Boston Scientific accounts are for healthcare professionals only.
Editorial commentary
Although there are a variety of ways to treat patients with prostate cancer, many options — including radiation therapy — can come with side effects.1 As a urologist helping patients manage prostate cancer, my patients rely on me to not just treat the cancer, but to do so while minimizing side effects and helping to maintain or improve the patient’s quality of life. To do so, I partner closely with the patient’s entire care team and incorporate procedures and technology that I have found to be reliable in my practice.
At Exeter, our patient care team includes a patient navigator who escorts the patient through the process, myself — the urologist — and a radiation oncologist who not only administers the radiation treatment but helps me counsel and educate patients about potential side effects. These side effects can vary widely and there’s a laundry list that we’re all familiar with, from bowel and urinary complications to sexual dysfunction.1,2
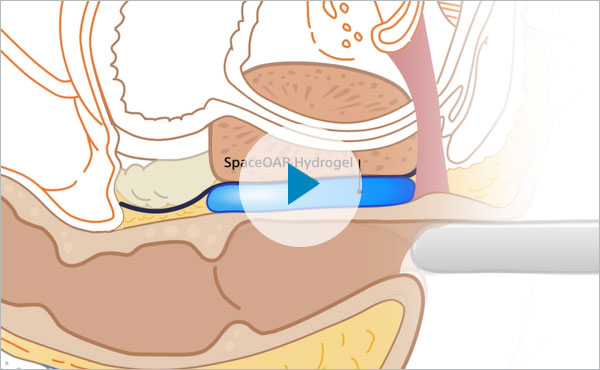
In my experience, one of the best ways to mitigate side effects from radiation is to place a SpaceOAR Hydrogel — a rectal spacer — to create temporary space between the prostate and rectum during radiation therapy. By creating this space, studies demonstrated that SpaceOAR Hydrogel resulted in 73% less rectal radiation and 75% less late rectal toxicity.2,3 Also, studies show that many patients experienced improved bowel quality of life, long-term compared to patients who did not receive a perirectal spacer.4
I also find it reassuring that there have been more than 220,000 SpaceOAR Hydrogel units shipped globally for prostate cancer patients since launching in 2015 and that number continues to grow.5
Considering the side effects and long-term impact of radiation
SpaceOAR Hydrogel creates a temporary space between the rectum and the prostate, allowing us to deliver radiation therapy to the prostate to treat the cancer while reducing radiation to the rectum. The importance of maximizing the cancer treatment while minimizing the long-term impact of radiation cannot be overstated.6 Potential side effects when radiating the prostate include, but are not limited to, painful urination, bladder pain, blood in the urine or stool, fecal leaking or incontinence, and erectile dysfunction.7 SpaceOAR Hydrogel serves as a way to potentially reduce those side effects.2
Placement of fiducial markers allows us to create a tomographic map to ensure that radiation is delivered precisely every time.8 Many urologists combine the placement of fiducial markers and SpaceOAR Hydrogel in the same procedure.2,9,10 I find that when I combine these two relatively simple procedures it is well tolerated by my patients.2,9
Many of my colleagues in urology have been following patients after radiation treatment for a number of years and we’ve seen patients experience the full gamut of side effects.1 Yet, I have personally been pleased with the results of SpaceOAR Hydrogel. I’ve had no complications in the delivery of SpaceOAR Hydrogel, and my patients have not had any complaints of radiation side effects. As someone who has used SpaceOAR Hydrogel for 5 years and who has treated scores of patients, I’m thankful to say that they’ve all done very well.
For example, one patient said that if it weren’t for SpaceOAR, he would have remained conflicted about his treatment choice for prostate cancer. He had medical conditions that precluded surgery and it was SpaceOAR that finally helped him feel good about pursuing radiation. Radiation delivered tremendous success without any side effects, and he is so thankful.
SpaceOAR Hydrogel placement procedure
When adopting SpaceOAR Hydrogel technology into my clinical practice, I was able to gain the knowledge and experience I needed by completing the required training and certification process with representatives from Boston Scientific. Their focus on education and the level of clinical support they provided were invaluable. In addition to providing an onboarding guide, Boston Scientific reps came to my practice and conducted a thorough in-service training program for office staff, clinical staff, and physicians — which took about an hour. Beyond our onboarding, they have continued to provide excellent support.
This was important to me as patient safety is always my primary focus. And before starting a new procedure, it’s vital that I and my entire team feel that it’s safe and have confidence in the process and technology and will have support if and when needed.
In my experience, the procedure itself is very straightforward and easy to manage. My experience with prostate biopsies and both needle guidance and ultrasound visualization has helped me to perform this procedure. In the pivotal trial, both the radiation oncologists and urologists who applied the spacer rated the device’s ease of use as “easy” or “very easy” 98.7% of the time.2 Given the upside of reducing the radiation to the rectum during radiation therapy, I would advise that every urologist consider it. Additionally, Boston Scientific allows physicians to utilize their proprietary equipment, including ultrasound, free for 90 days or 10 procedures, so the investment during training and evaluation period is minimal.
Hydrodissection: Placement and when to abort the procedure
A critical first step and required as part of the Instructions for Use for SpaceOAR Hydrogel, is to start with hydrodissection. It guides the placement of the spacer and ensures that the needle tip is in the correct location — the perirectal fat — and not in the periprostatic fascia, Denonvilliers’ fascia, or rectal wall.5
Hydrodissection is relatively quick, it typically takes me from about 30 seconds to 3 or 4 minutes, depending on the patient’s anatomy.6 It involves confirming placement of the needle which can be seen in the sagittal and axial views on a sonogram to verify proper positioning. Once I have confirmed I’m in the right place, I hydrodissect and then do a quick aspiration to make sure the needle is not in a blood vessel. This would be exceedingly rare, but it can happen, and in that case, I would discontinue the procedure.
It's important to not proceed with gel placement if you’re not comfortable with the needle tip placement during this step. In fact, there are instances where I abort a case at this stage. If there’s abundant gas or stool in the rectum, limiting my vision and impacting placement of the needle, then I reconsider. There are also patients who have paucity of perirectal fat, which is also challenging. If the hydrodissection doesn’t go as expected, or I’m unable to place the tip of the needle in that thin elusive perirectal fat (it may be too anterior in the Denonvilliers’ fascia or too posterior in the perirectal wall), then I abort.
SpaceOAR Vue: Improving workflow efficiencies
Another option — which I prefer for my patients — is using the next-generation SpaceOAR Vue™ Hydrogel. This is an iodonized hydrogel spacer that offers enhanced visibility via CT scan compared to non-iodinized spacers.5 That’s the paramount reason to use SpaceOAR Hydrogel Vue, but it’s also cost effective for patients to only have a post-procedural CT scan as opposed to an additional MRI.11–13
Urologists and their office staff go through many steps to obtain pre-authorization for MRIs.11–13 So being able to streamline and avoid the MRI by using SpaceOAR Vue Hydrogel can help achieve overall efficiency. Simply put, it’s easy to perform, easy to schedule, and easy to review because urologists are generally very good at reading CT scans. SpaceOAR Vue Hydrogel allows me to evaluate — in multiple phases and multiple planes — the effect of the spacer regardless of whether we’re looking at axial, coronal, or sagittal images. My office now has a protocol to include a CT scan after every SpaceOAR Vue Hydrogel placement and that has improved our workflow efficiencies because we don’t have to determine which patients cannot have an MRI.
Conclusion
I truly believe that SpaceOAR Hydrogel is one of the best ways to help reduce the side effects of radiation during prostate cancer treatment.2 SpaceOAR Vue Hydrogel is the next-generation hydrogel that is designed to offer similar clinical benefits that SpaceOAR Hydrogel provides. I’ve had no complications in the delivery, and my patients have not had any rectal complaints. Overall, my patients have done very well with SpaceOAR Hydrogel, and I think it should be considered for all patients embarking on a radiation pathway.
Related content
References
- American Cancer Society, Radiation Therapy for Prostate Cancer. Available at: https://www.cancer.org/cancer/prostate-cancer/treating/radiation-therapy.html. Accessed December 1, 2022.
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–977.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985.
- Miller LE, Efstathiou JA, Bhattacharyya SK, Payne JA, Woodward E, Pinkawa M. Association of the placement of a perirectal hydrogel spacer with the clinical outcomes of men receiving radiotherapy for prostate cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e208221.
- Data on file with Boston Scientific.
- Chen HHW, Kuo MT. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget. 2017;8:62742–62758.
- Mayo Clinic. External Beam Radiation for Prostate Cancer. https://www.mayoclinic.org/tests- procedures/external-beam-radiation-for-prostate-cancer/about/pac-20384743. Accessed December 2021.
- Memorial Sloan Kettering Cancer Center. About Your Prostate Fiducial Marker Placement. https://www.mskcc.org/cancer-care/patient-education/about-your-prostate-fiducial-marker-placement. Accessed October 17, 2022.
- Brenneman RJ, Goddu SM, Andruska N, et al. Feasibility of same-day prostate fiducial markers, perirectal hydrogel spacer placement, and computed tomography and magnetic resonance imaging simulation for external beam radiation therapy for low-risk and intermediate-risk prostate cancer. Pract Radiat Oncol. 2022;12:e117–e122.
- Navaratnam A, Cumsky J, Abdul-Muhsin H, et al, Assessment of polyethylene glycol hydrogel spacer and its effect on rectal radiation dose in prostate cancer patients receiving proton beam radiation therapy. Adv Radiat Oncol. 2019;5:92–100.
- Gold J. With medical debt rising, some doctors push for payment upfront. NPR. https://www.npr.org/sections/health-shots/2014/04/25/303720114/with-medical-debt-rising-some-doctors-push-for-payment-upfront. Published April 25, 2014. Accessed July 20, 2023.
- LaVito A. Two in three patients can't pay off their hospital bills. CNBC Health and Science. https://www.cnbc.com/2017/06/26/two-in-three-patients-cant-pay-off-their-hospital-bills.html. Published June 26, 2017. Accessed July 20, 2023.
- Olen H. Even the insured often can't afford their medical bills. The Atlantic. https://www.theatlantic.com/business/archive/2017/06/medical-bills/530679/. Published June 18, 2017. Accessed July 20, 2023.
E. William (Bill) Johnson, M.D., is a Boston Scientific consultant and was compensated for his contribution to this editorial. Statements made represent the opinion and experience of Dr. Johnson. Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
IMPORTANT INFORMATION: These materials are intended to describe common clinical considerations and procedural steps for the use of referenced technologies but may not be appropriate for every patient or case. Decisions surrounding patient care depend on the physician’s professional judgment in consideration of all available information for the individual case.
Boston Scientific (BSC) does not promote or encourage the use of its devices outside their approved labeling. Case studies are not necessarily representative of clinical outcomes in all cases as individual results may vary.
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
For information purposes only. The content of this article/publication is under the sole responsibility of its author/publisher and does not represent the opinion of Boston Scientific.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.