Despite improvements in radiotherapy treatment techniques, GI toxicity can be a significant concern for patients undergoing prostate cancer treatment. Late GI toxicity is associated with factors commonly encountered in the prostate cancer patient population, namely advanced age and existing comorbidities.17 Late GI toxicity is associated with the maximal rectal dose and volume of the anterior rectal wall irradiated, which can significantly decrease patient-reported quality of life.4,15,16,18–21 Patient-reported outcomes are valuable metrics for assessing these side effects that often occur long after treatment ends.22,23 Some approaches to increase patient convenience include using hypofractionated and ultra-hypofractionated (i.e., stereotactic body radiation therapy, SBRT) regimens, which are associated with comparable biochemical recurrence-free survival but may still be associated with increased late GI toxicity.24–29 Fortunately, there are techniques that can limit the exposure of healthy GI tissue during radiation therapy for prostate cancer.
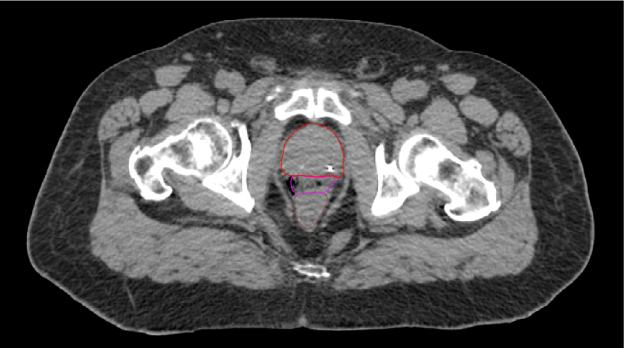
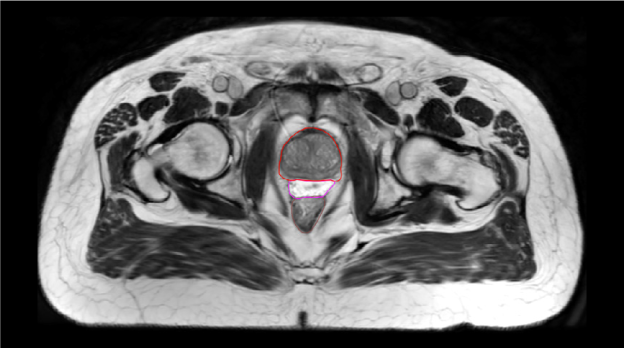
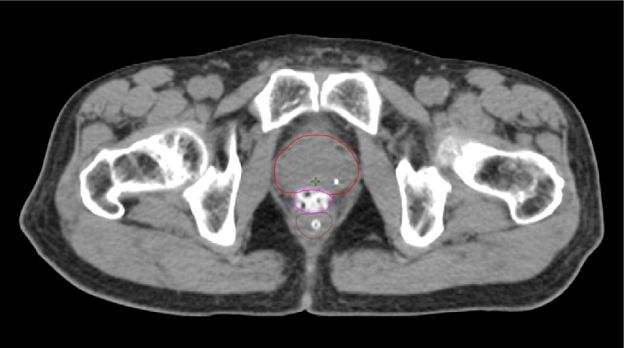
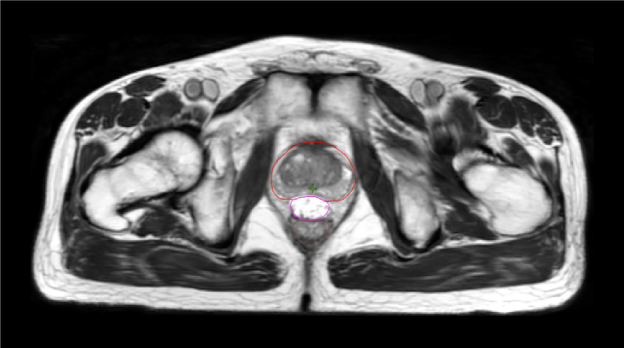
One pre-radiation approach to reduce radiation-associated rectal toxicity is to physically increase the distance between the rectum and prostate in a temporary manner.30 The anterior rectal wall is typically within 2–3 mm of the posterior prostate border and is separated from the prostate and bladder by a single multi-layered fibromuscular fascia (i.e., Denonvilliers’). This tissue plane is a potential space that can be artificially expanded using exogenously introduced material such as rapidly polymerizing hydrogel.5 In the authors’ opinion, artificial expansion of the perirectal space prior to starting radiotherapy should:
- Be reproducible
- Be easily and safely performed in the outpatient setting with minimal time investment
- Cause minimal to no patient discomfort while in place
- Remain stable throughout the course of radiotherapy planning and treatment
- Have minimal to no adverse effect on daily setup and treatment
- Biodegrade within a reasonable timeframe following completion of therapy
SpaceOAR™ Hydrogel (OAR = organs at risk) (Boston Scientific, Marlborough, MA) was designed to address these listed characteristics of an ideal spacer. Approved by the Food and Drug Administration (FDA) as well as CE marked, the SpaceOAR System is intended to temporarily displace the anterior rectal wall so as to reduce radiation exposure during prostate cancer radiotherapy treatment. Recently, a second-generation hydrogel spacer, SpaceOAR Vue™ Hydrogel, was developed by Boston Scientific that uses the characteristics of traditional SpaceOAR Hydrogel, while providing a contrast agent designed to be easily tracked by CT imaging. The purpose of this paper is to describe SpaceOAR Hydrogel and to highlight potential key benefits of SpaceOAR Vue Hydrogel for clinicians interested in using a hydrogel spacer for patients undergoing radiation therapy for prostate cancer.