CAUTION: TheraSphere is under an investigational device exemption for treatment of patients with metastatic colorectal cancer. The safety and effectiveness for this treatment has not been established.
EPOCH trial overview
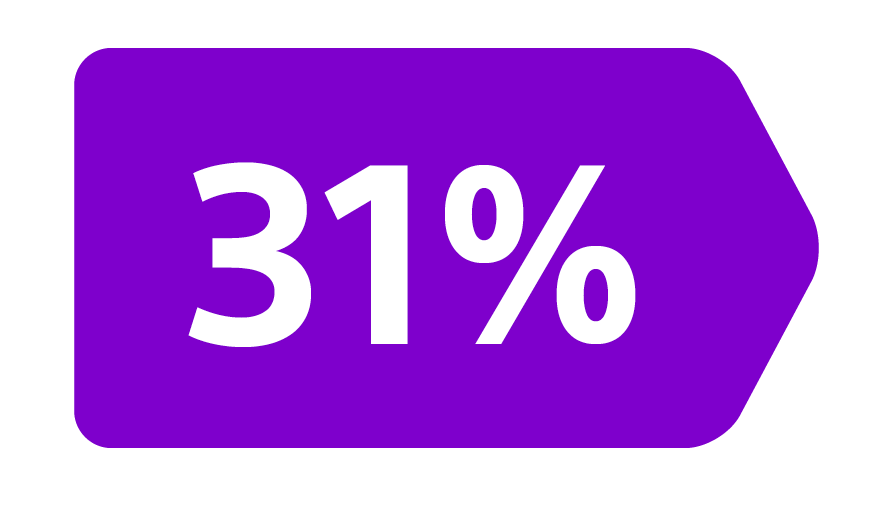
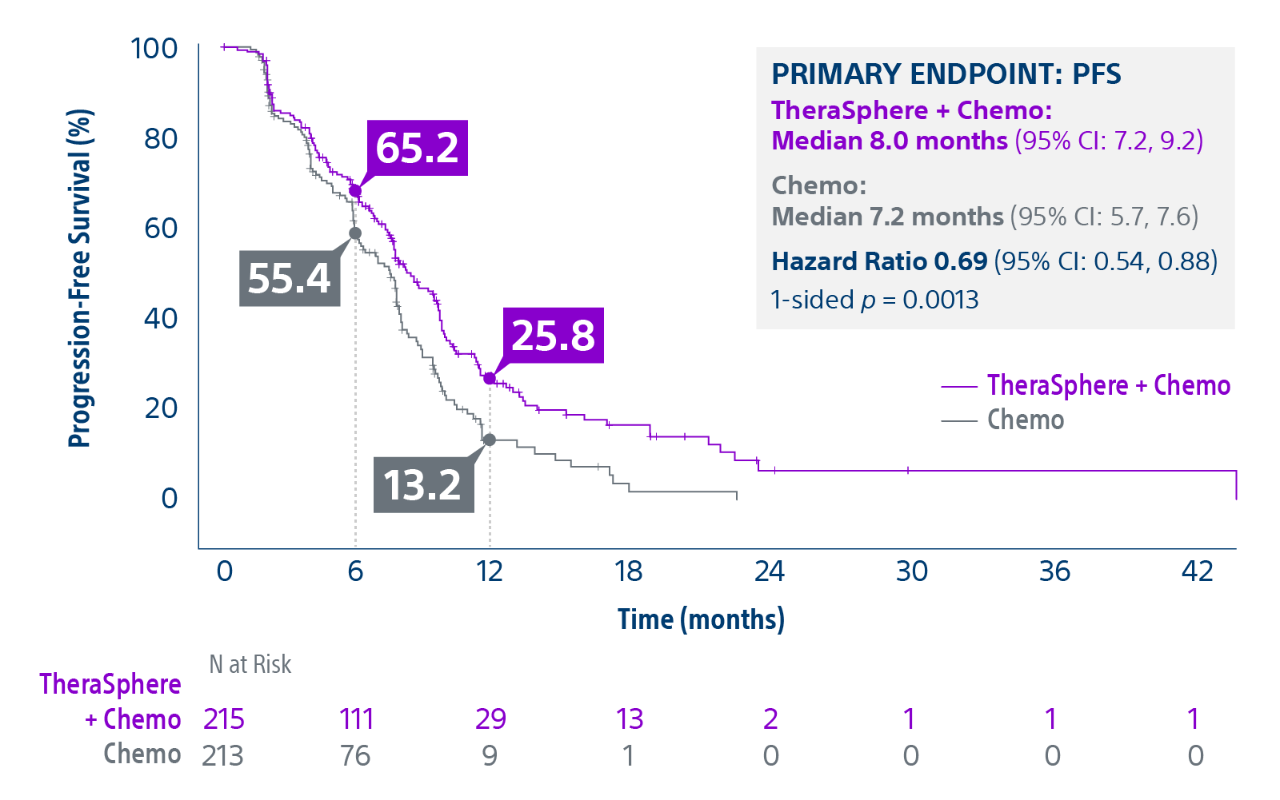
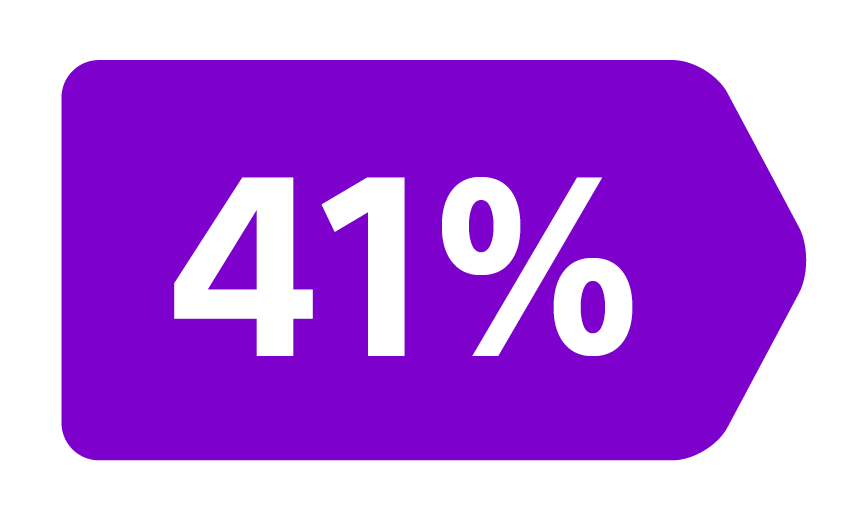
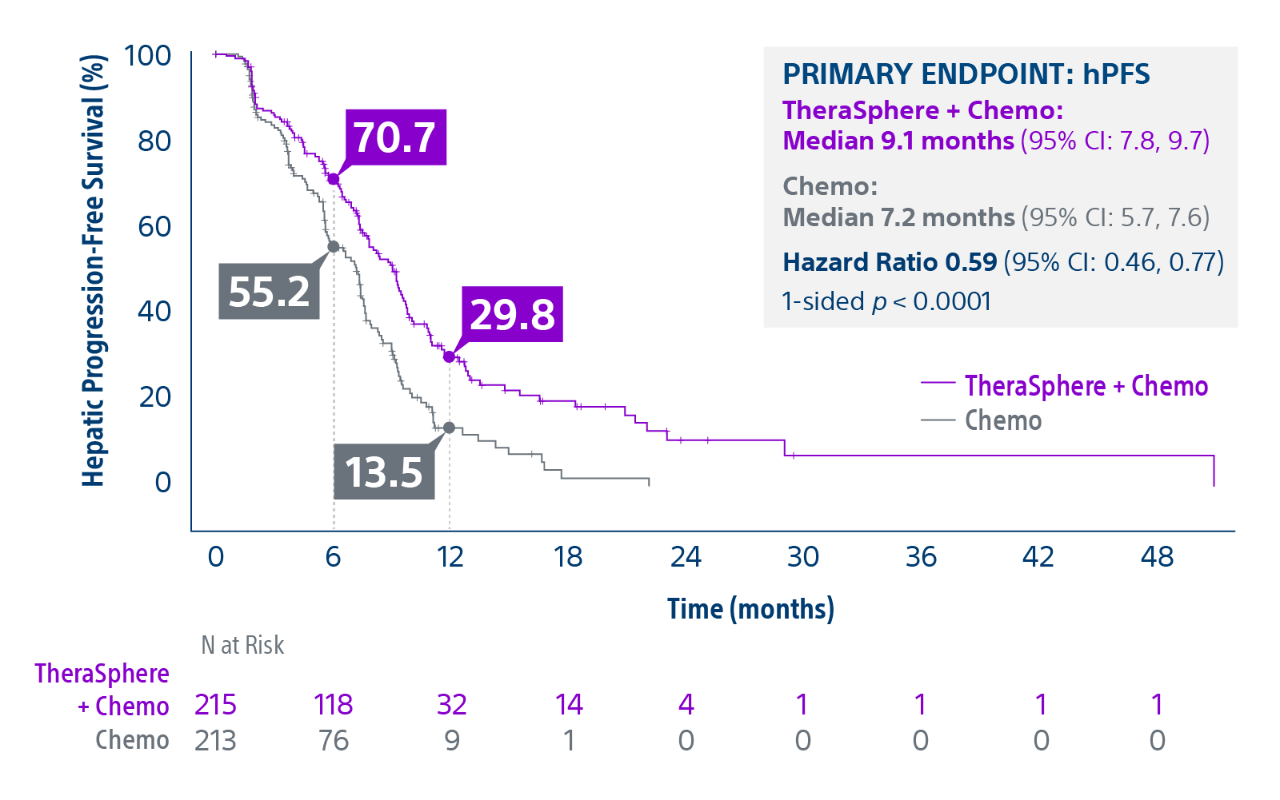
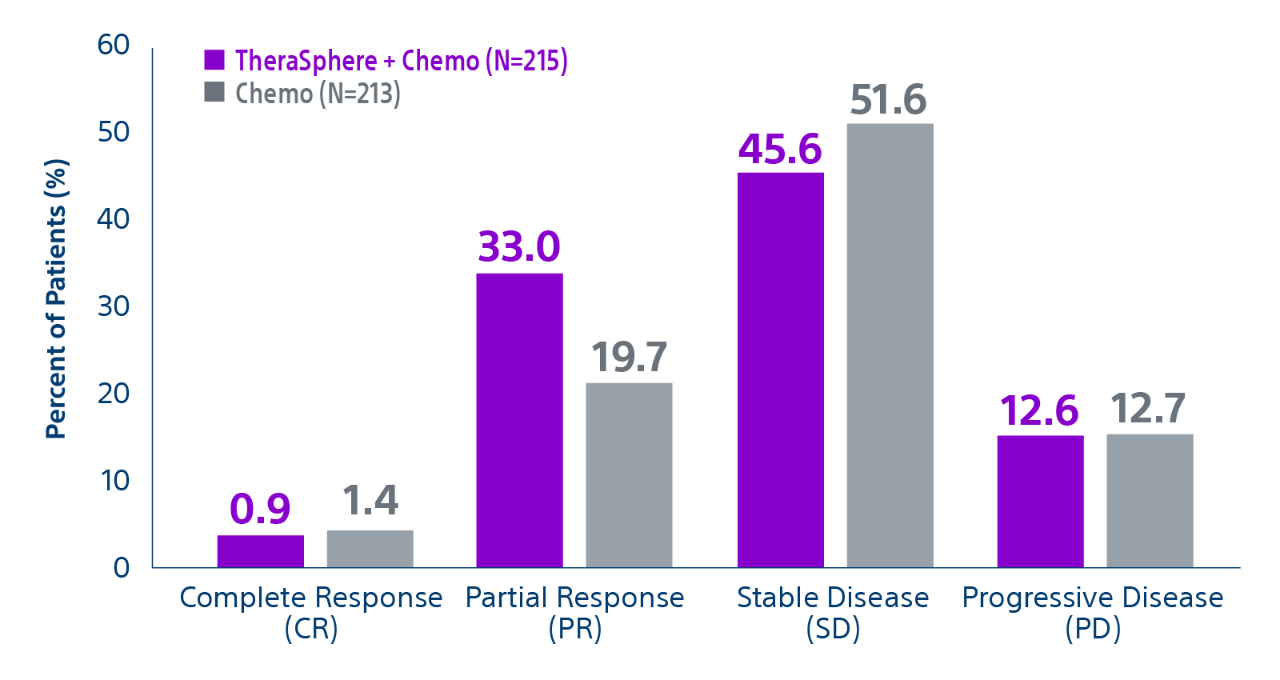
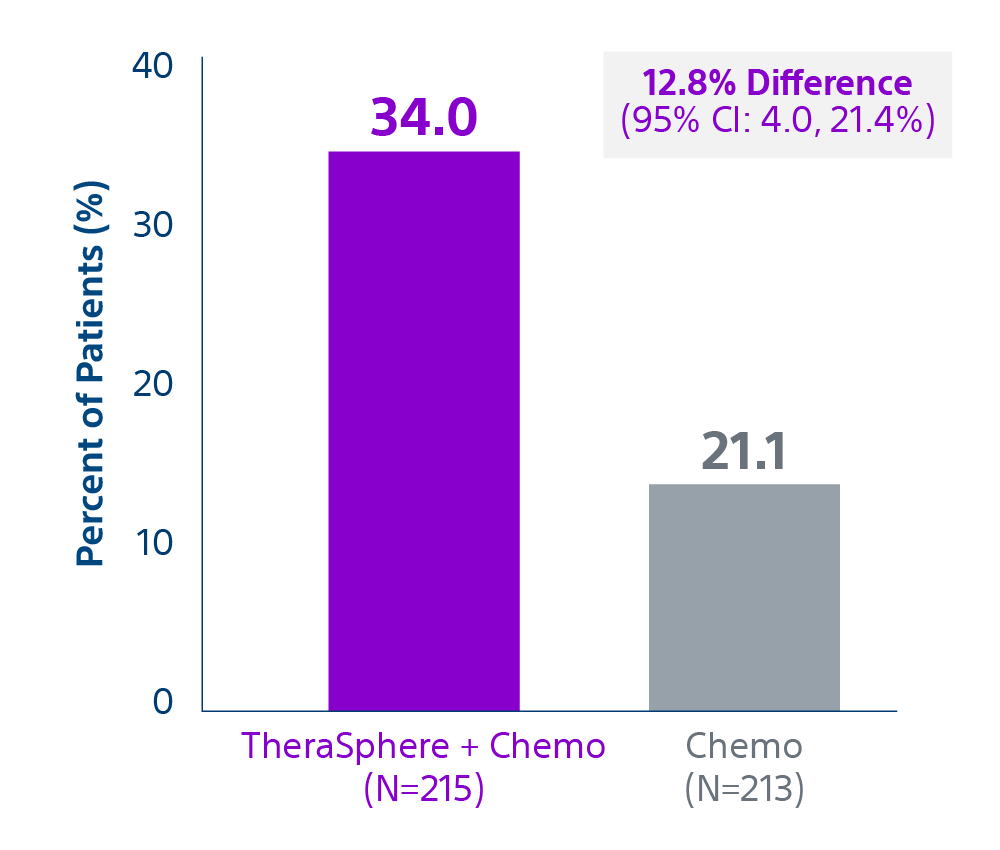
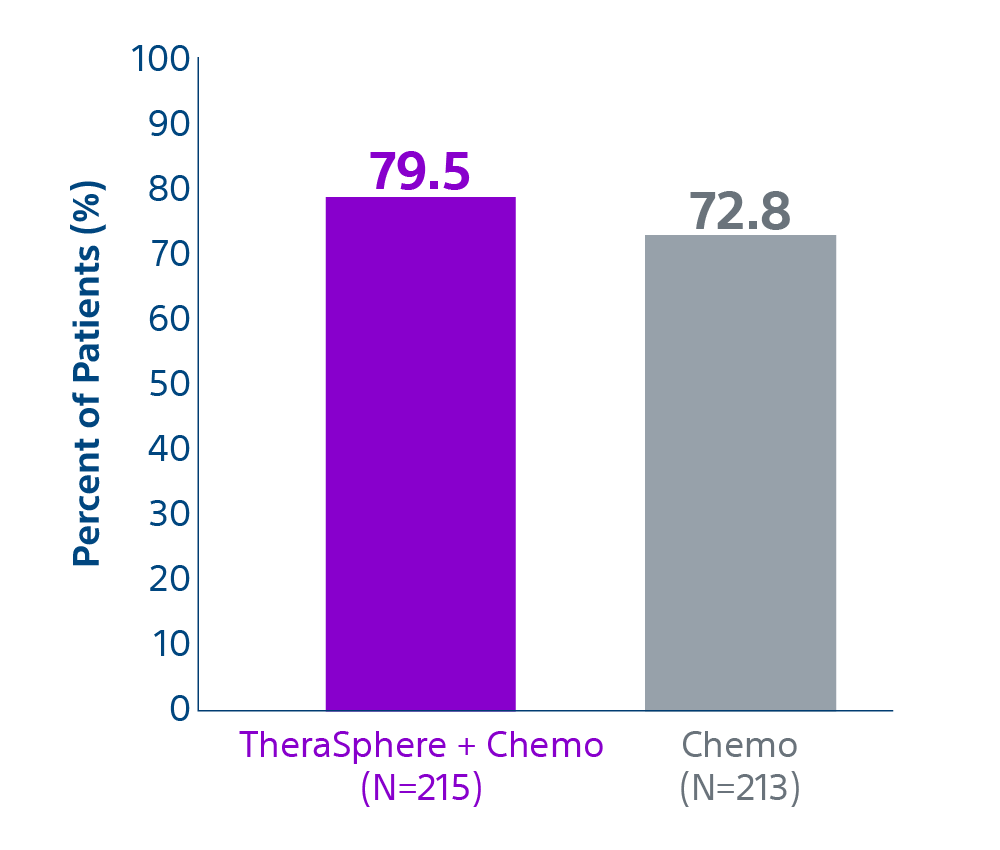
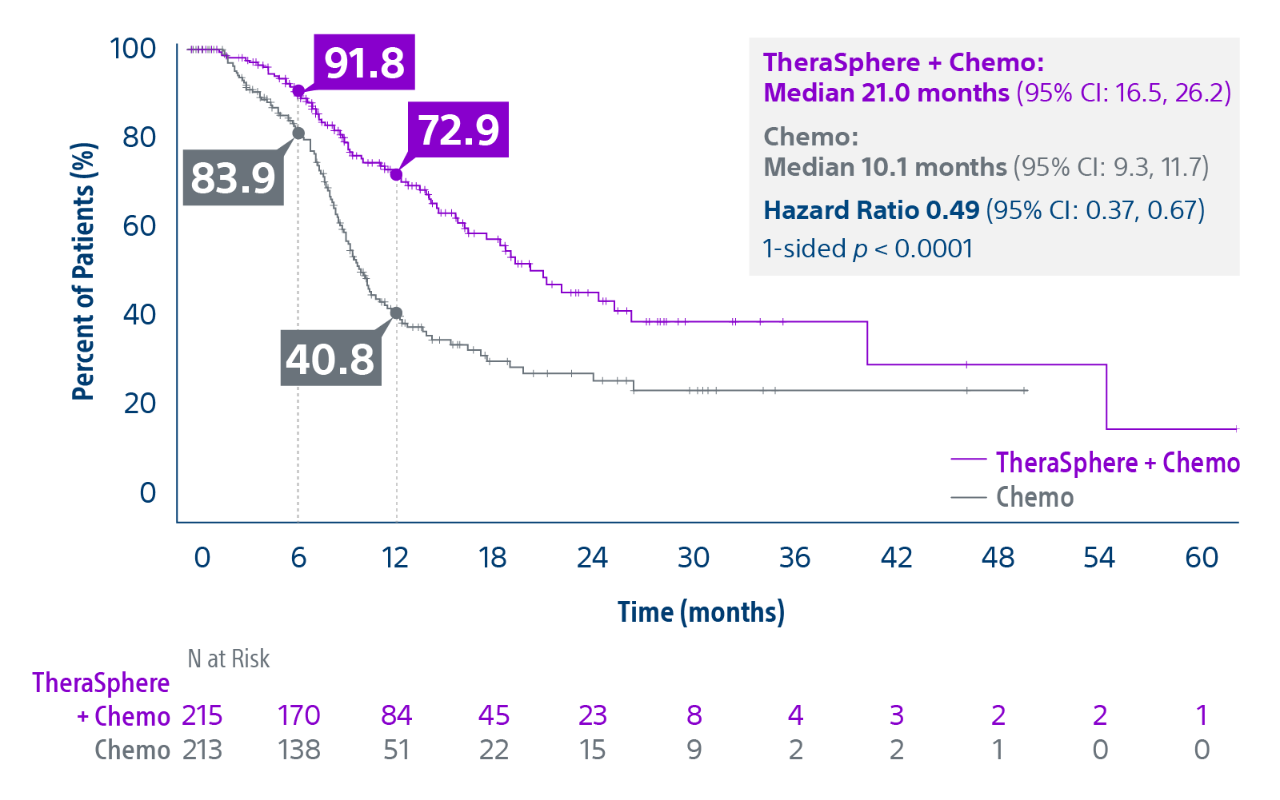
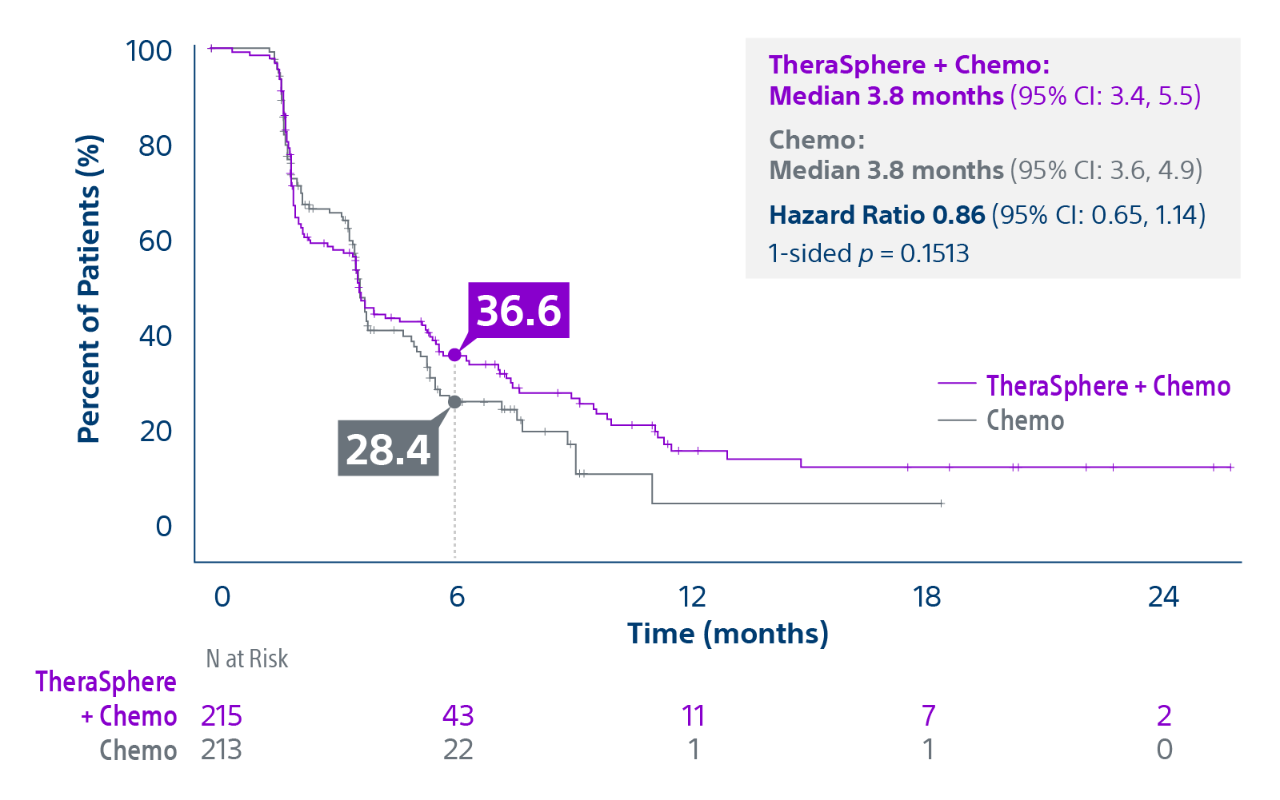
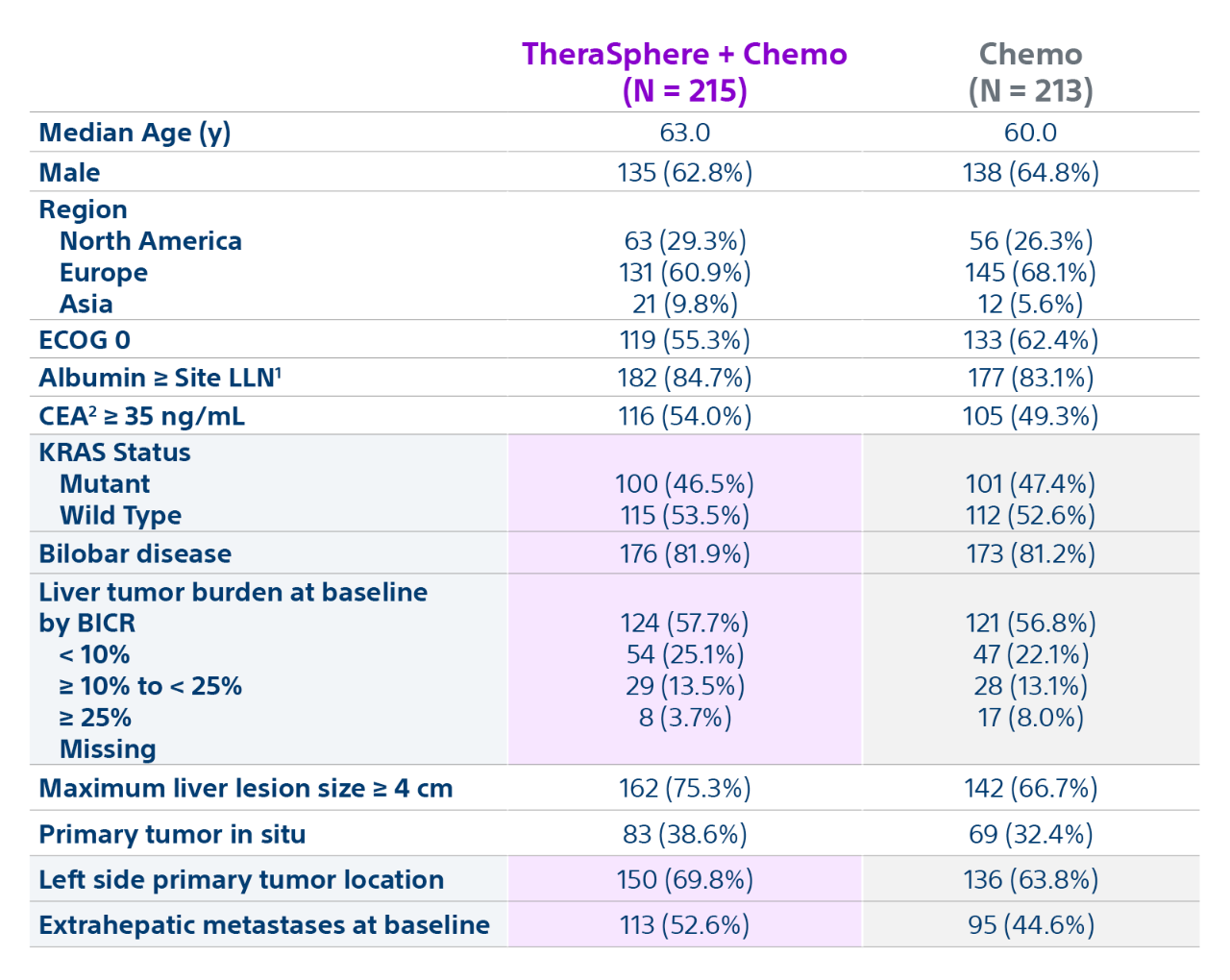
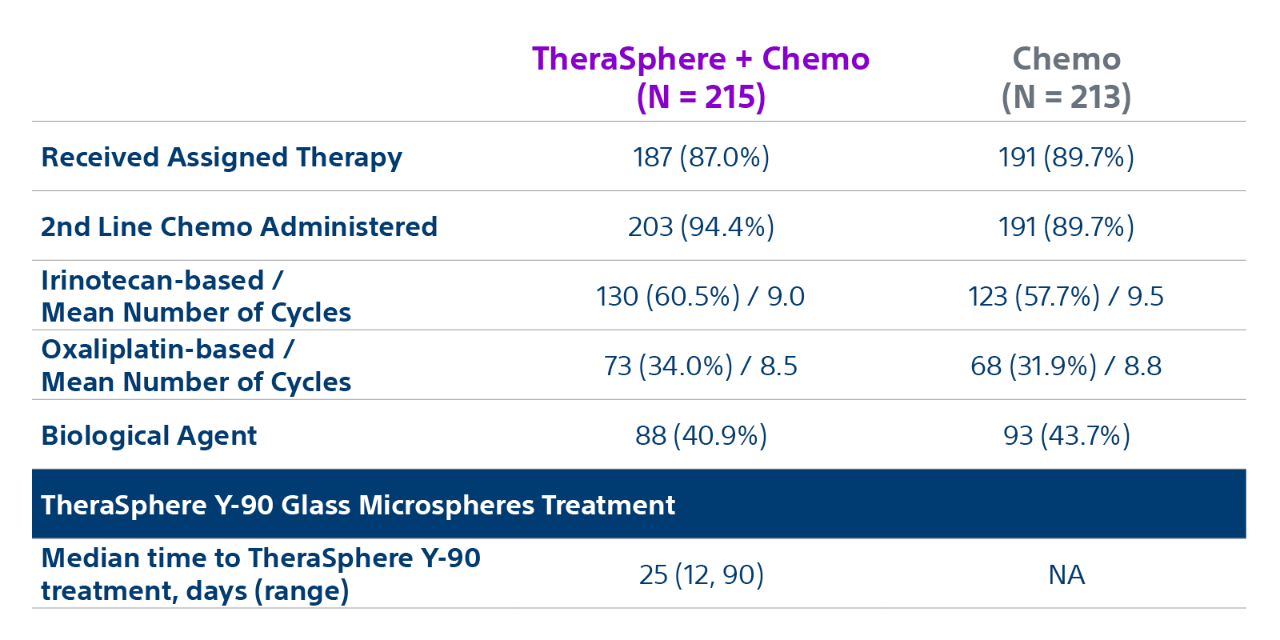
Epoch is a level 1, phase III randomized controlled trial using transarterial radiation therapy for mCRC liver metastases that demonstrated statistically significant improvements in both Progression-Free Survival (PFS) and Hepatic Progression-Free Survival (hPFS) in patients who progressed on first-line chemotherapy.¹