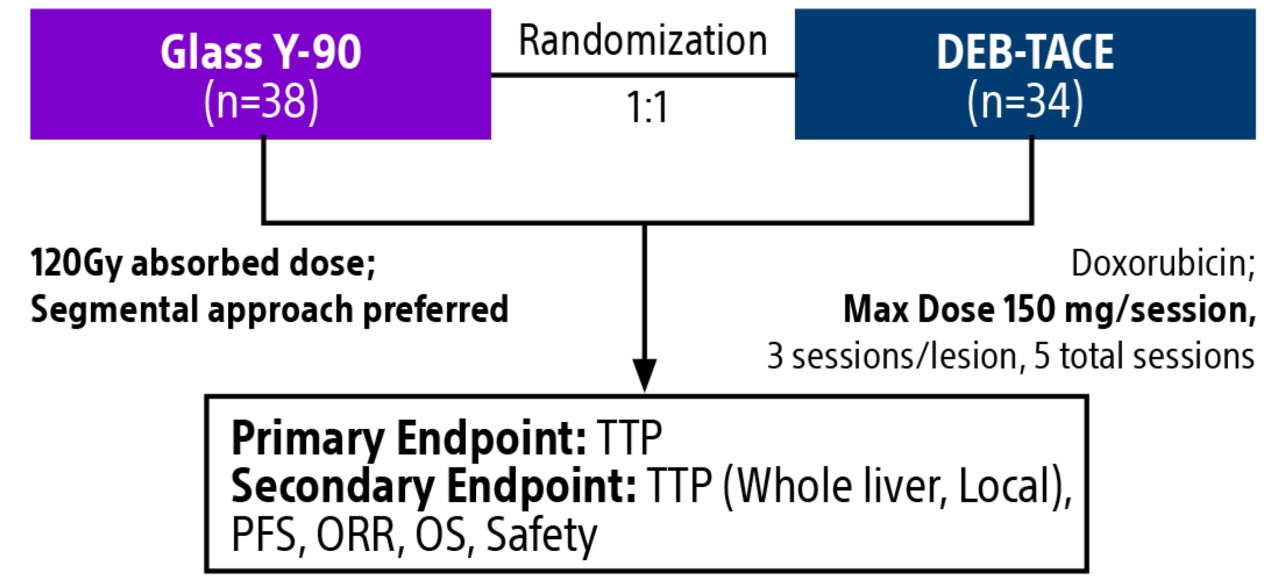
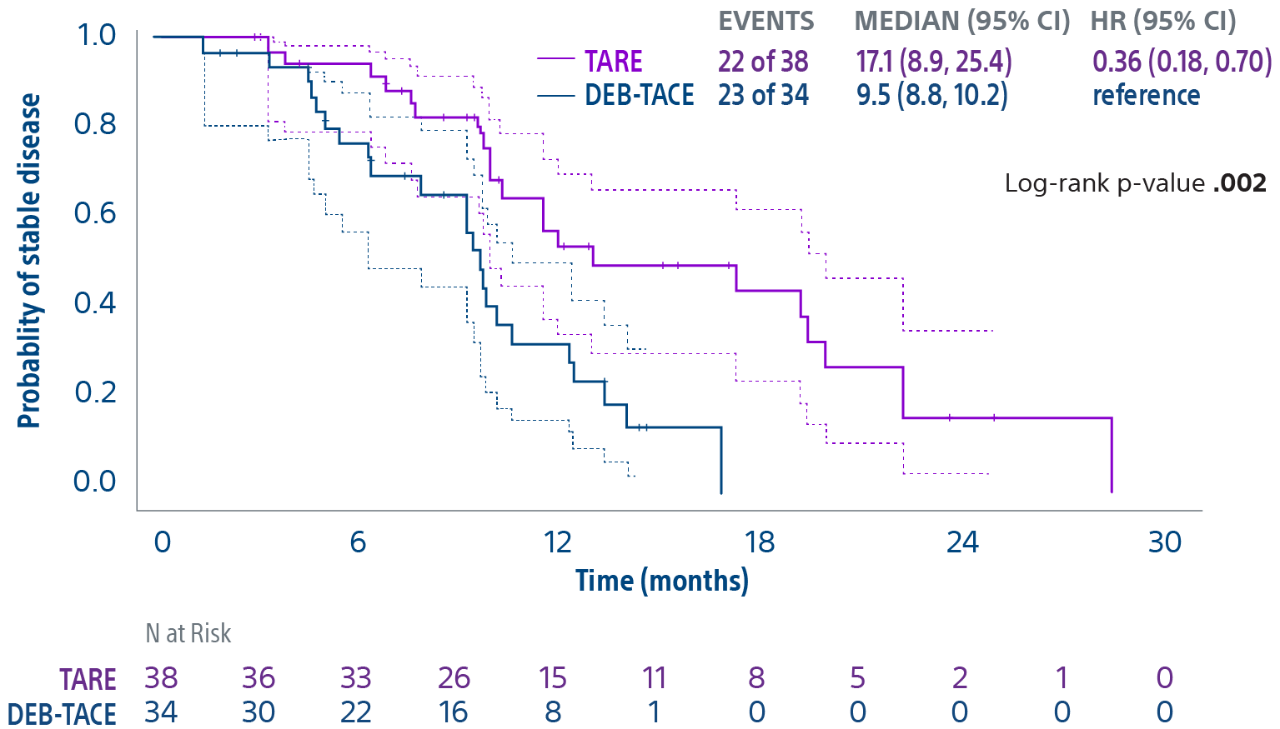
TACE & Y-90: PREMIERE & TRACE trials overview
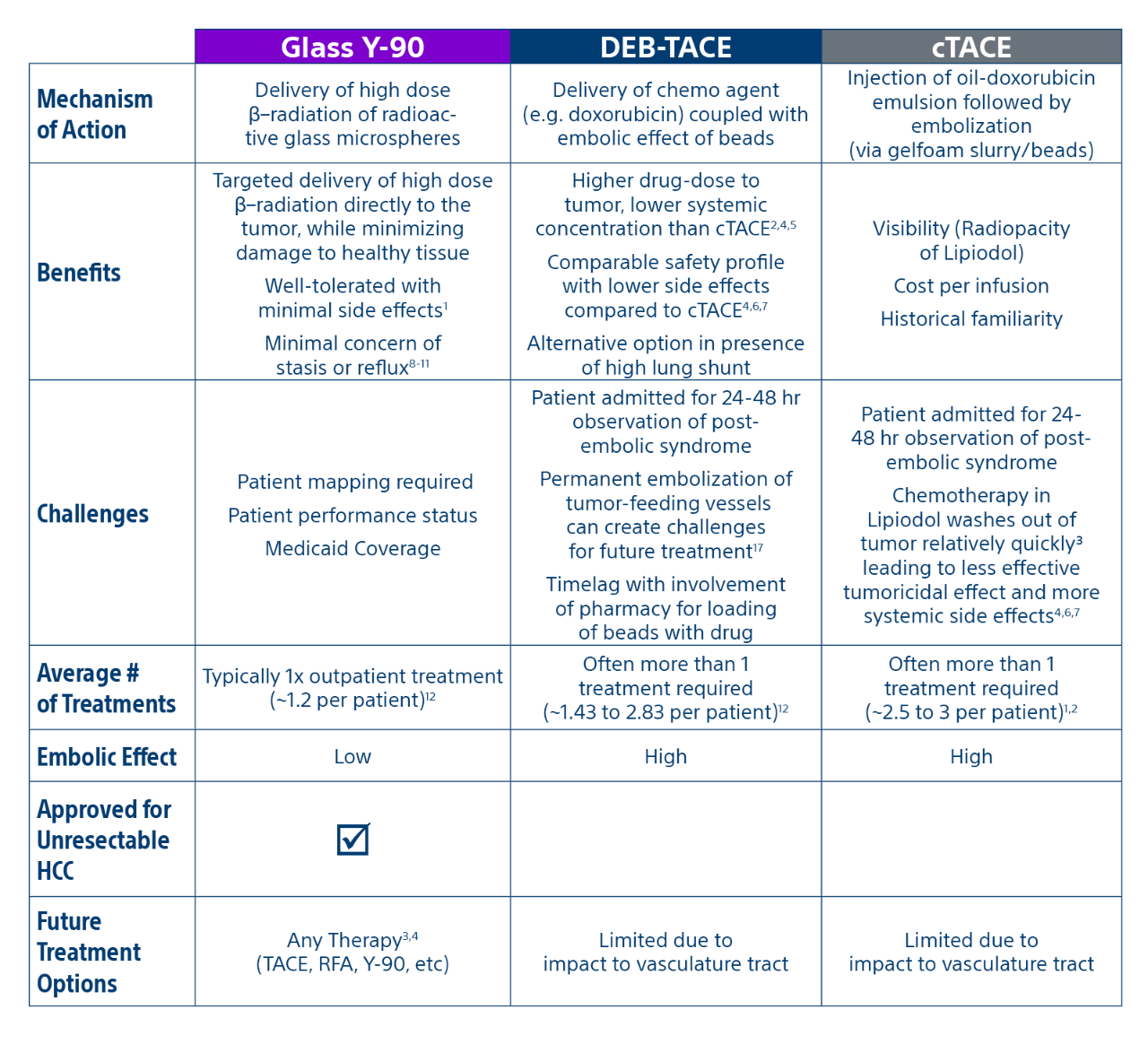
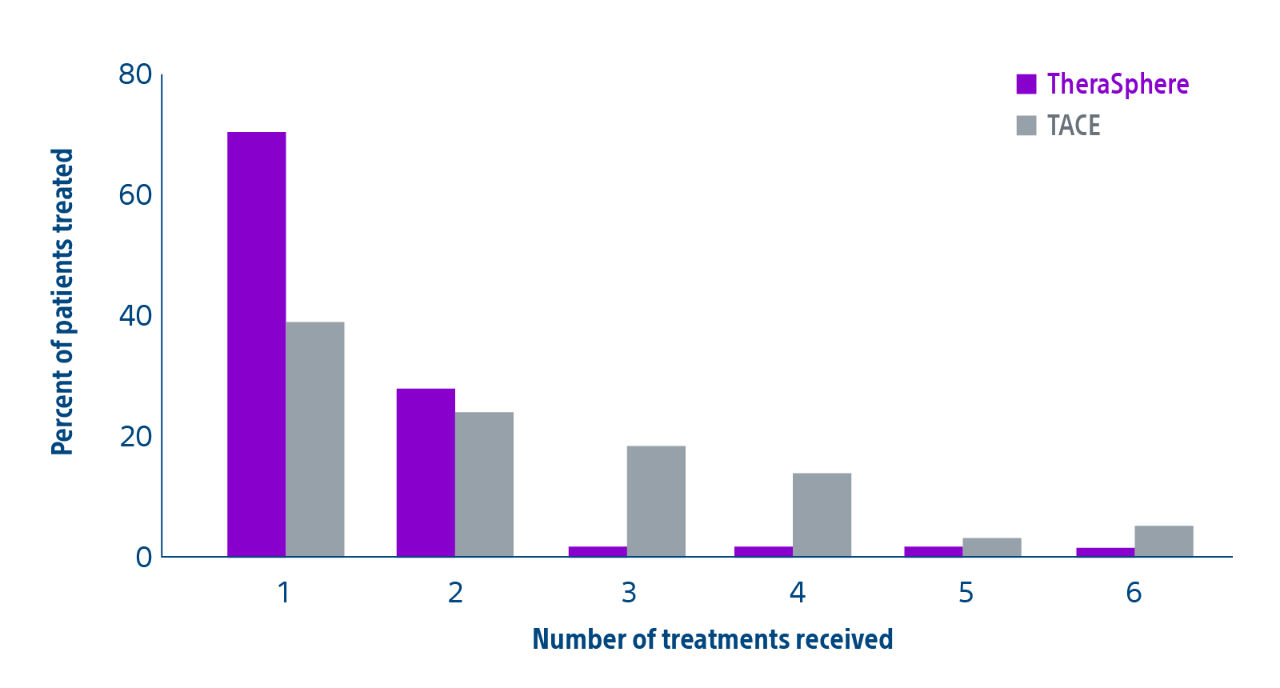
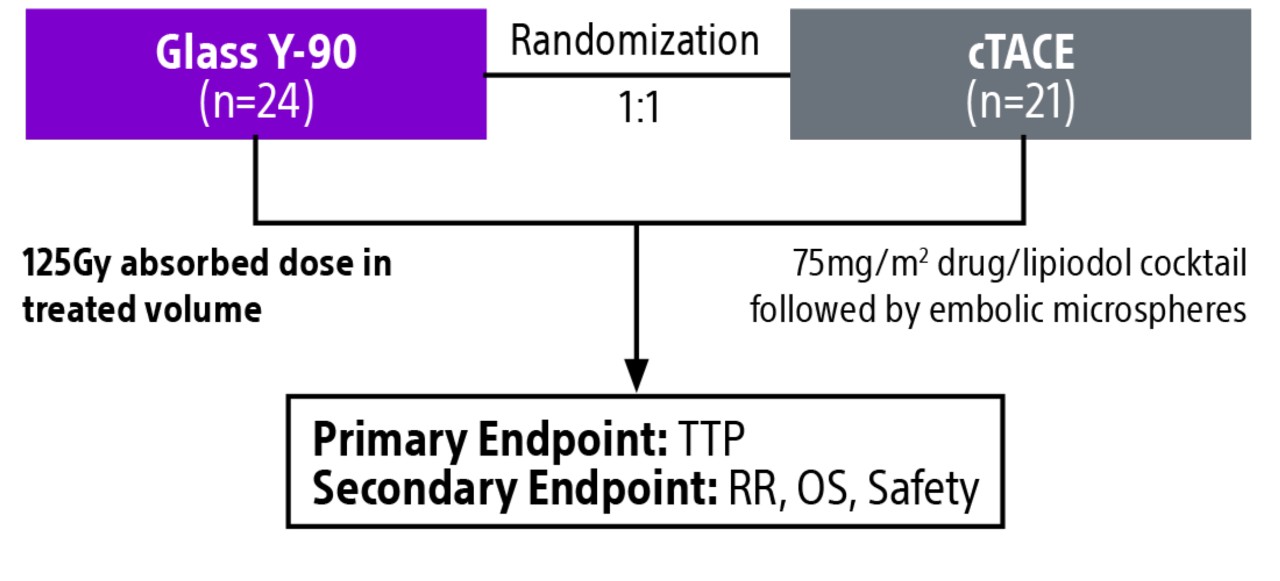
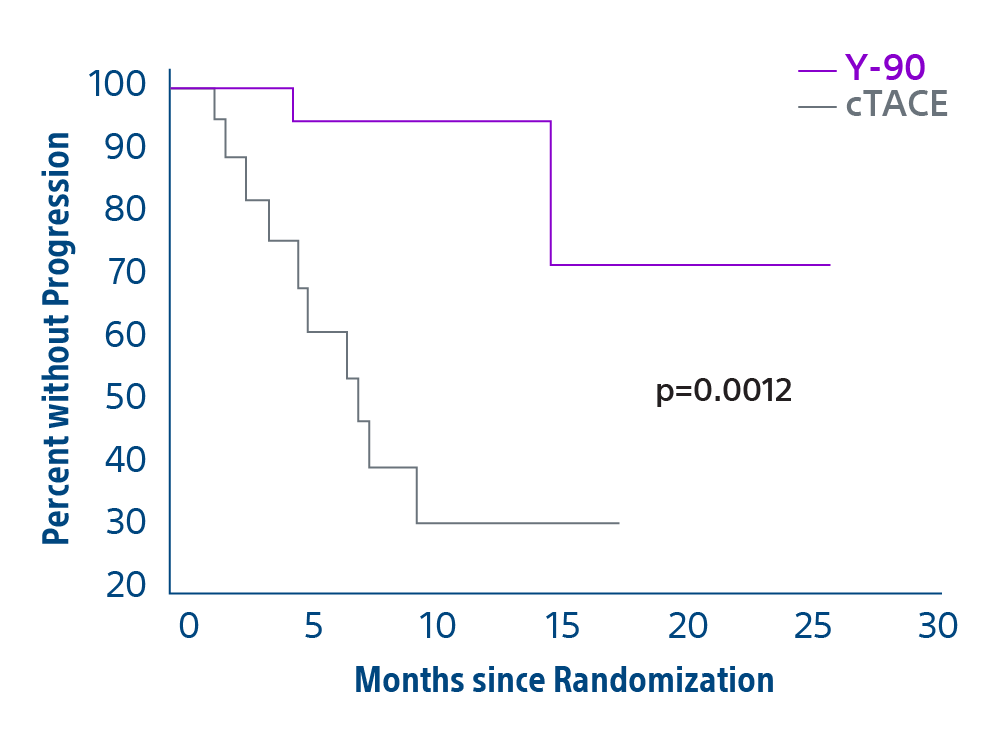
Comparison of contemporary transarterial radioembolization (TARE) treatment via TheraSphere to drug-eluting bead transarterial chemoembolization (DEB-TACE*) and conventional transarterial chemoembolization (cTACE) in patients with HCC shows longer time to tumor progression as a cost-effective, efficient treatment option.
*The loading of doxuribicin to LC Beads is outside of the indication for use in the USA.