DOSISPHERE-01 overview
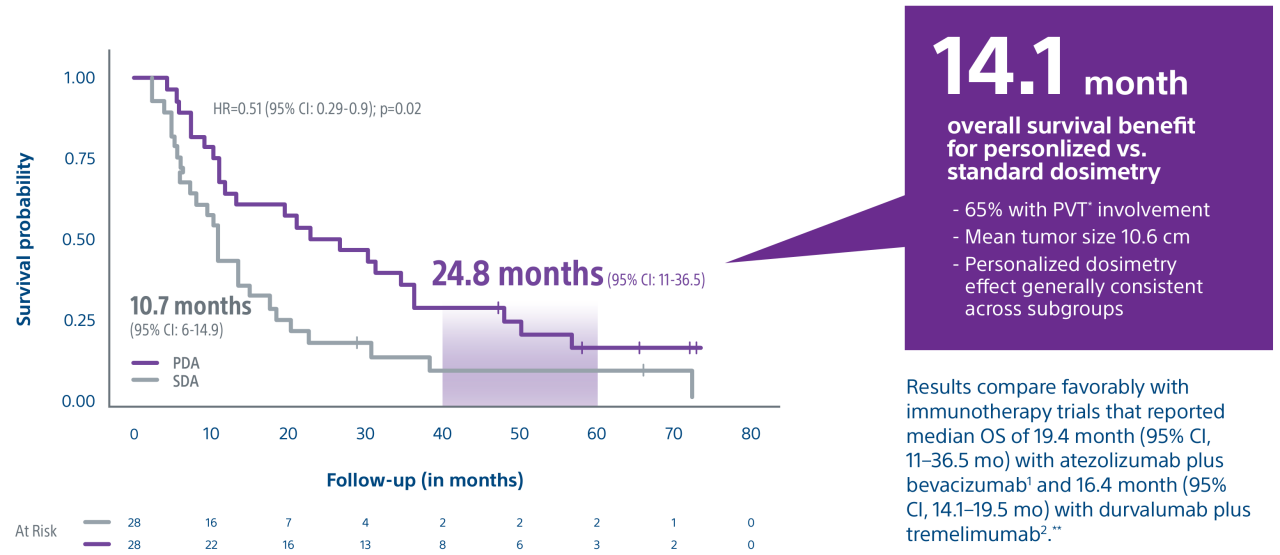
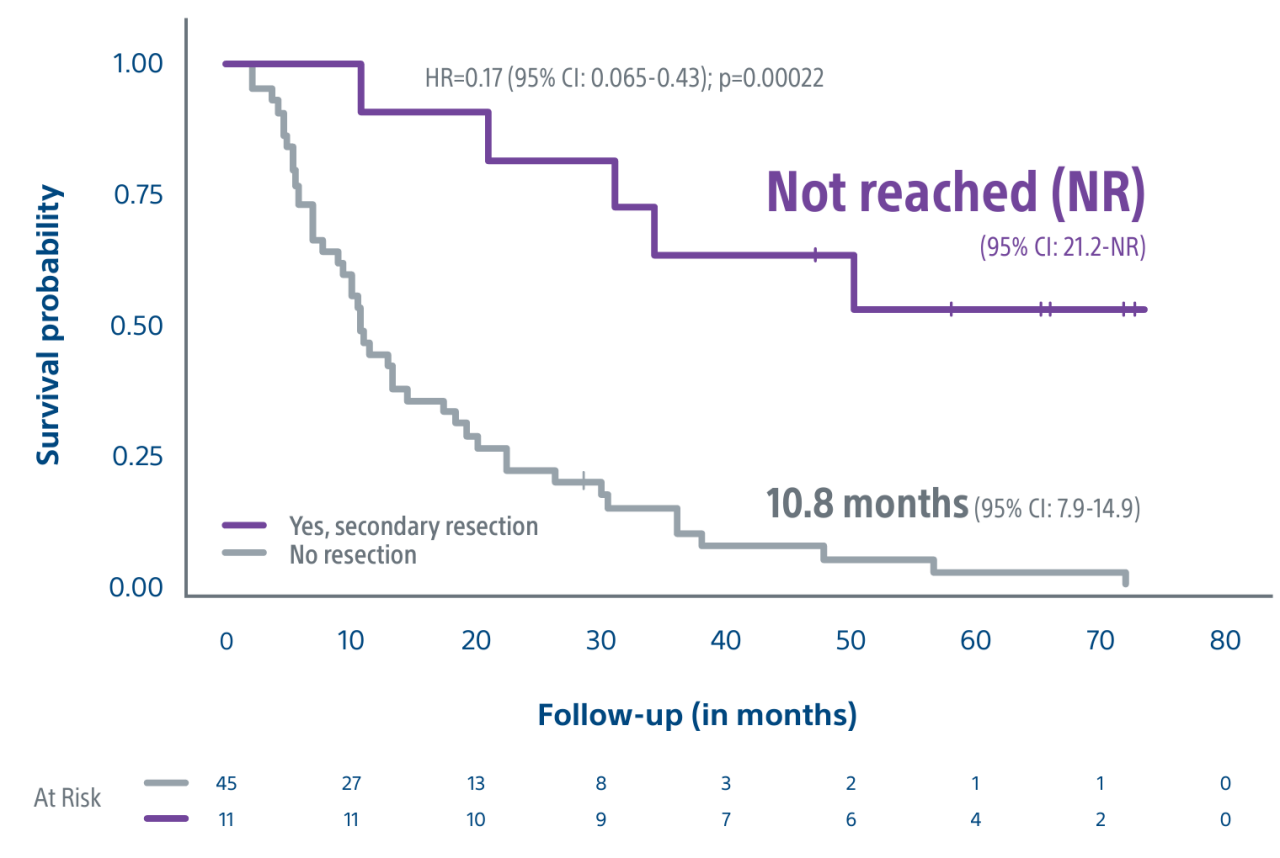
Level 1 evidence showed that unresectable HCC patients who received a personalized TheraSphere dose using multi-compartment dosimetry and were downstaged to resection saw durable, long-term overall survival.
Garin E, Tselikas L, Guiu B et al. Personalized versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021, 6: 17-29.
Garin E, Tselikas L, Guiu B, et al. Long-Term Overall Survival After Selective Internal Radiation Therapy for Locally Advanced Hepatocellular Carcinomas: Updated Analysis of DOSISPHERE-01 Trial. J Nucl Med. 2024;65(2):264-269. Published 2024 Feb 1. doi:10.2967/jnumed.123.266211
Study objective
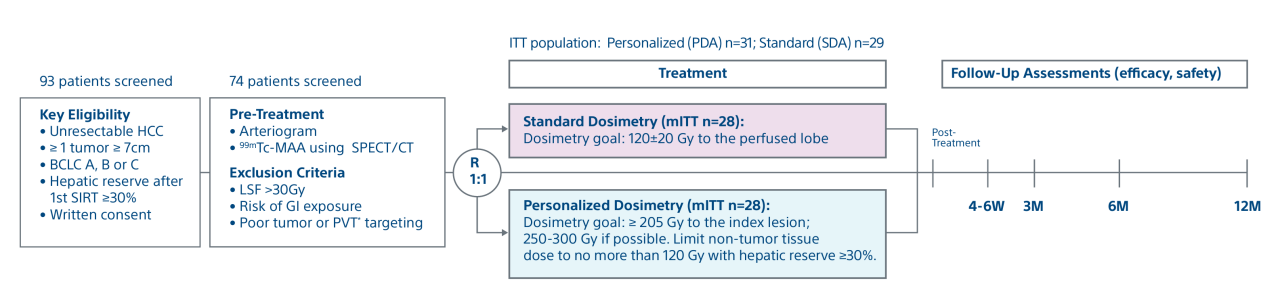
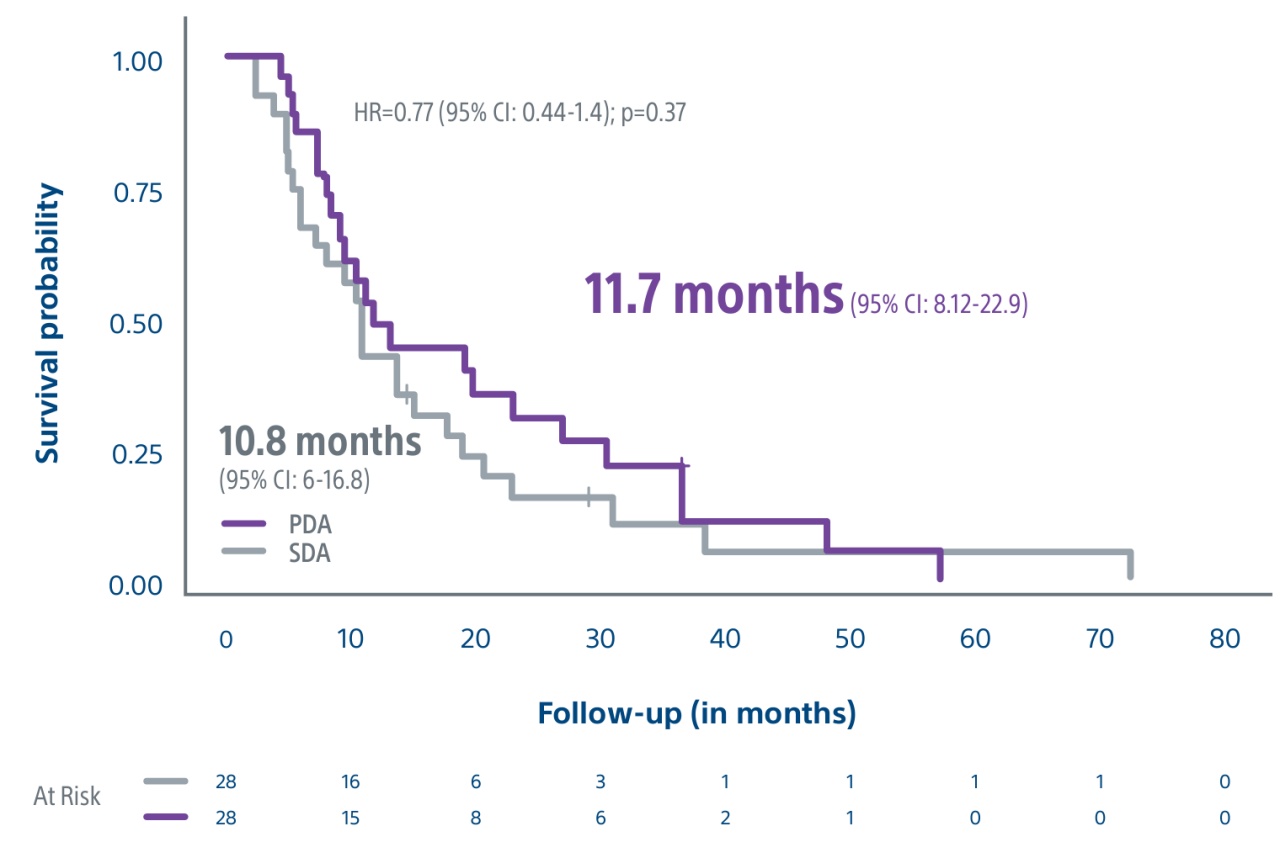
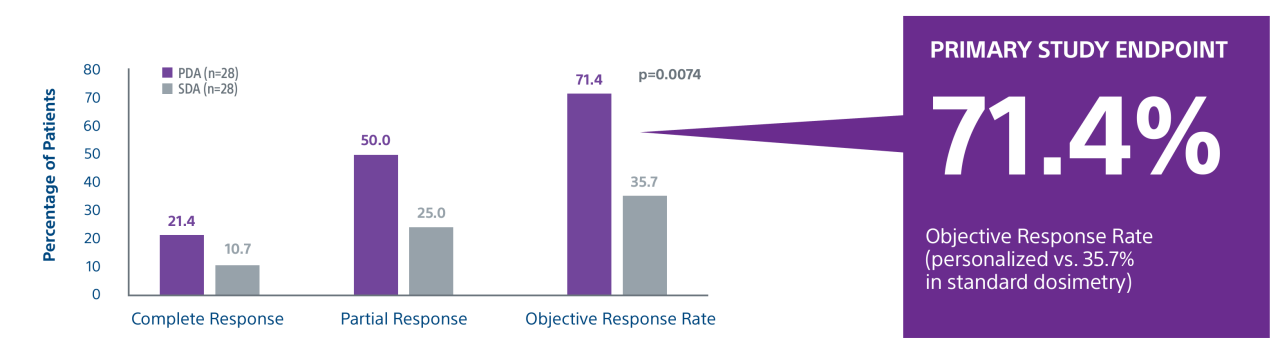
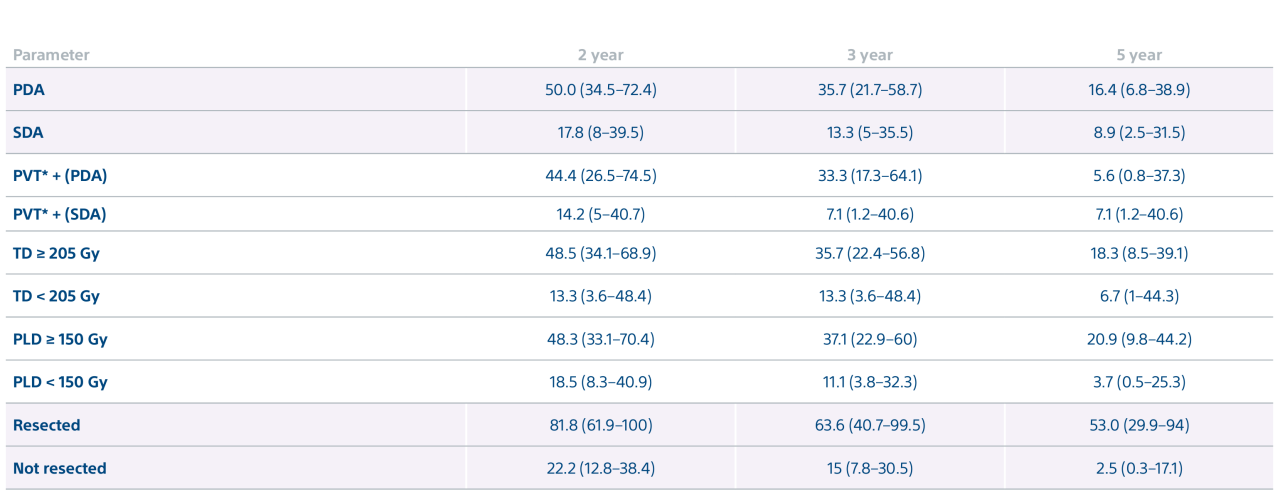
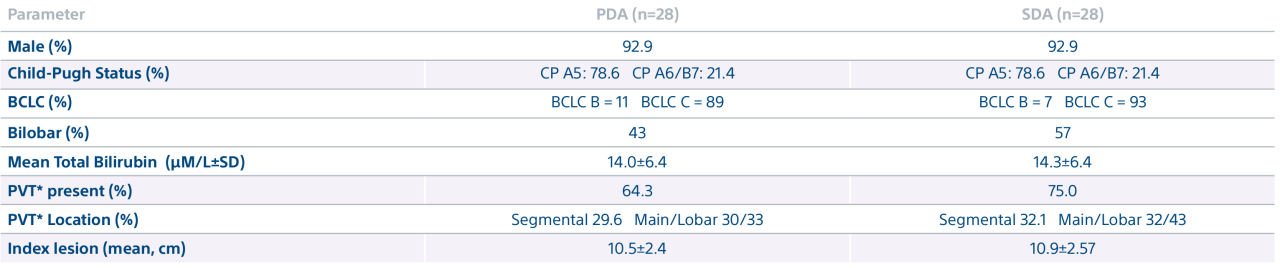
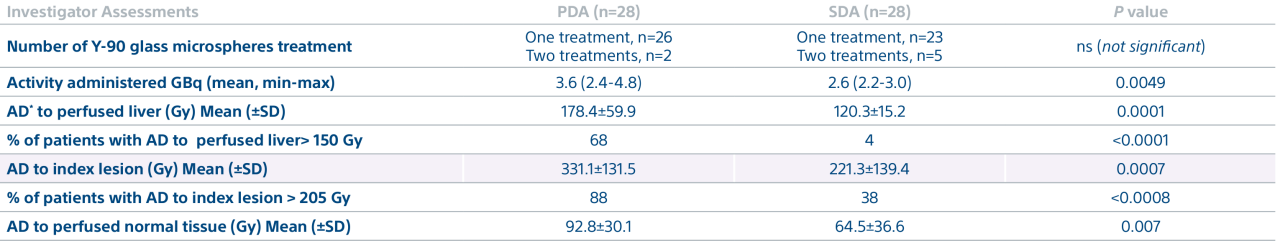
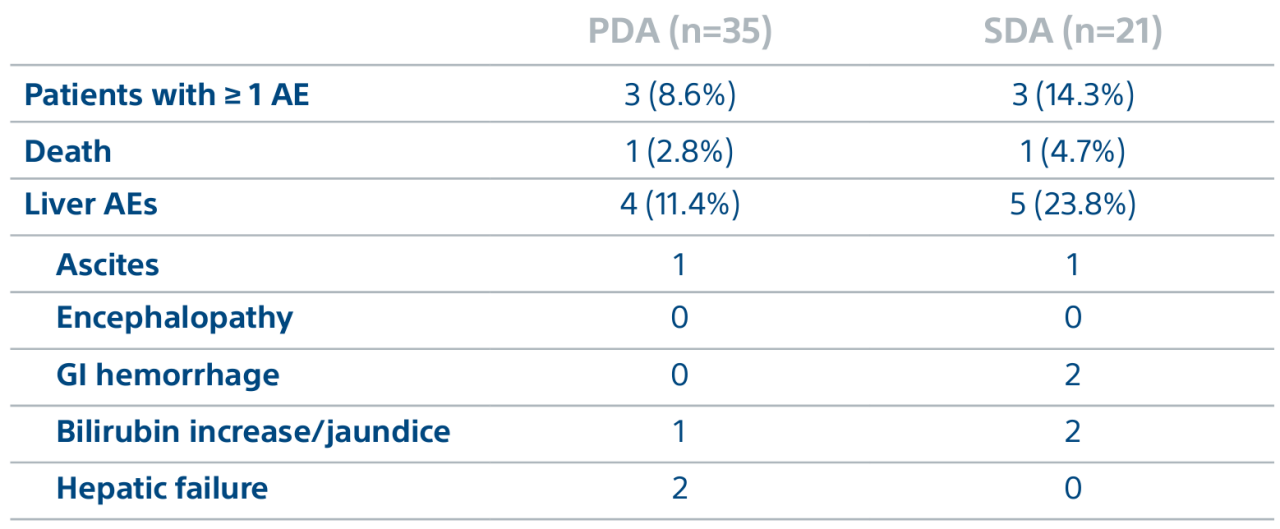
A randomized, multicenter, investigator sponsored phase II trial comparing the clinical outcomes of SIRT with TheraSphere in patients with intermediate/advanced HCC using two pre-treatment dosimetry determination methods: (1) Standard, single-compartment dosimetry (SDA); defined as a uniform distribution of absorbed dose within the perfused volume – both tumor and normal liver or (2) Personalized dosimetry (PDA); defined as multi-compartment Y-90 distribution of absorbed dose within the perfused volume that accounts for preferential blood f low into the tumor compared with normal parenchyma.