Interventional technologies
Offering the broadest portfolio of therapies for peripheral artery disease, venous disease, and cancer uniquely positioned to help you:
- Improve clinical outcomes while reducing cost
- Achieve operational efficiencies
- Increase patient access to care
Bringing innovation in drug-eluting technologies, clot management, and interventional oncology.
- Peripheral artery disease
- Deep vein thrombosis
- Critical limb ischemia
- Pulmonary embolism
- Liver cancer
- Kidney & lung tumors
Evidence-backed drug-eluting portfolio
Eluvia™ Drug-Eluting Stent and RangerTM Drug Coated Balloon are the first devices for peripheral artery disease (PAD) backed by level one randomized controlled trials (RCTs).
Boston Scientific offers two drug-eluting solutions for PAD, both backed by head-to-head trials for comparative effectiveness. Physicians choose the treatment that is right for the lesion and the patient.
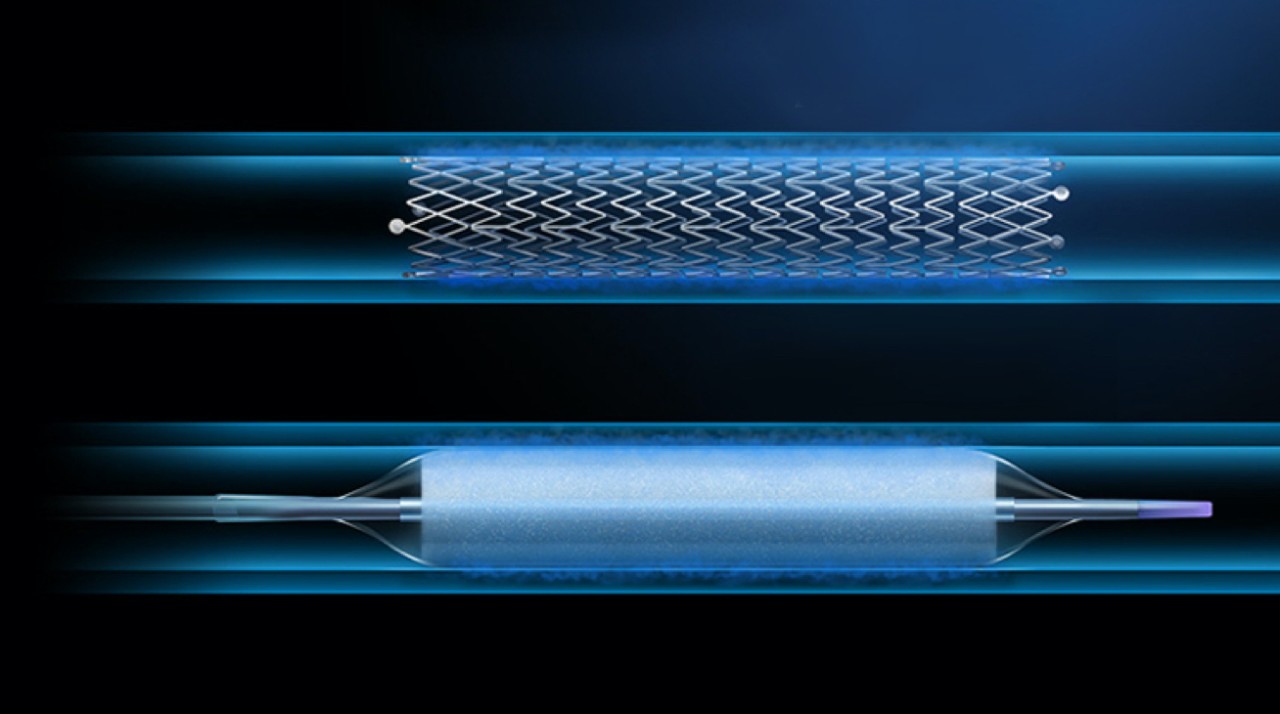
Top image: ELUVIA™ Drug-Eluting Stent
Bottom image: Ranger™ Drug-Coated Balloon
4
Randomized-controlled trials (RCTs) between Eluvia and Ranger
With a growing body of clinical evidence demonstrating exceptional outcomes, Boston Scientific is advancing science in the fight against peripheral artery disease.
Eluvia™ drug-eluting vascular stent system
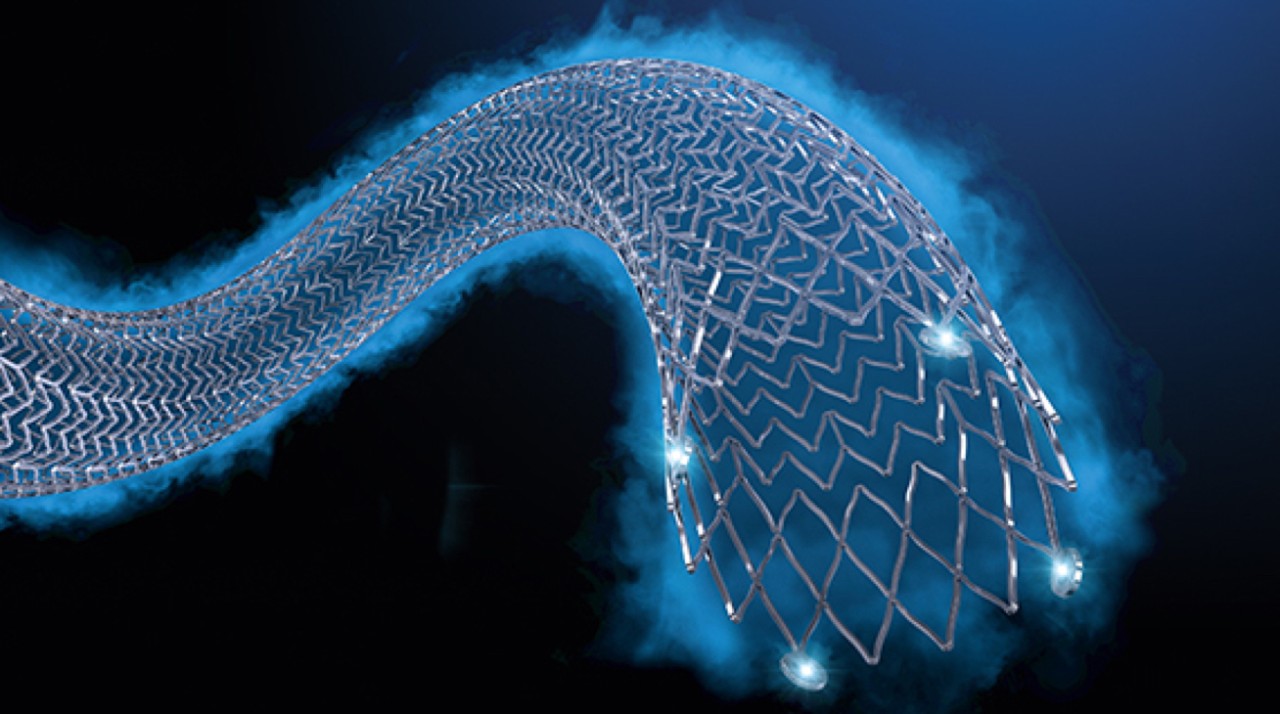
A new standard of care in SFA stenting
No SFA stent has performed better at 2-Years. No matter the lesion complexity. No matter the patient.1,2
| Superior patency | Statistically significant superior primary patency at 1-year over bare metal stents and Zilver™ PTX™ |
| Fewer repeat procedures | Statistically significant reduction in reinterventions at 2 years vs. Zilver™ PTX™5 |
| Shorter hospital stay | 19% reduction (>1 day) in average length of hospital stay vs. Zilver™ PTX™6 |
Ranger™ drug-coated balloon catheter
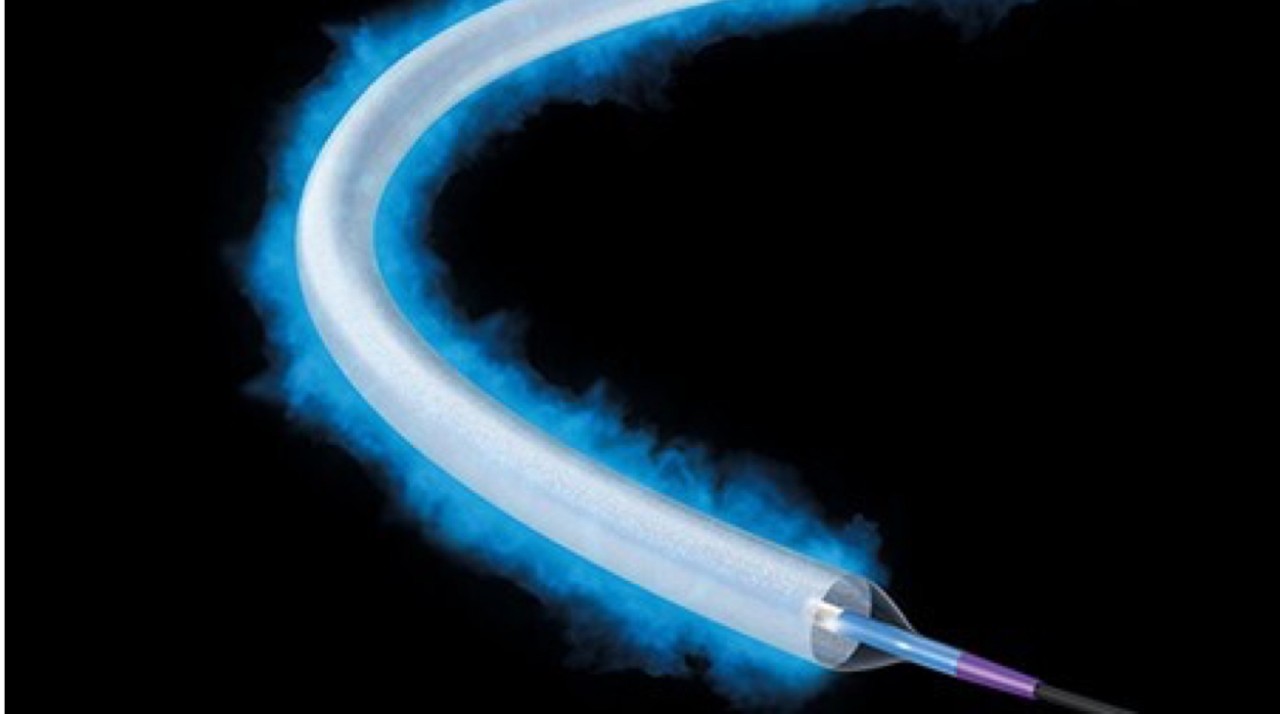
Exceptional Outcomes. Effortless Deliverability.
Backed by two randomized controlled trials, Ranger DCB consistently demonstrated high primary patency and low reintervention rates.7
Half
The total drug dose8 and similar primary patency as the IN.PACT DCB at 1 & 2-Years9
Lowest
Tip entry profile of any SFA DCB10 for a smooth delivery with excellent trackability
.014"/.018"
Guidewire compatibility can help streamline atherectomy procedures
The most studied and preferred tools for clot management
Boston Scientific is committed to delivering a full suite of interventional tools to target thrombus.
EKOS™ endovascular system
Ultrasound assisted catheter-directed thrombolysis
Dissolves clot with less lytic and short treatment times.
EKOS is clinically proven to be safe and reliable with long-term outcomes. No other device used to treat pulmonary embolism has been studied as much as EKOS. See the evidence.
~90%
Less thrombolytic dose than standard systemic treatment11,12
15 minute procedure that is easy to perform
14
Clinical trials published and >75,000 PE patients treated*
* Data on file
Evidence-based medicine, now backed by updated reimbursement. Reimbursement for the procedure codes applicable to the EKOS system increased October 1, 2020. To help your institution benefit from these changes, access the EKOS coding guide.
AngioJet™ peripheral thrombectomy system
Rapidly removes thrombus via mechanical thrombectomy with optional lytic delivery.
AngioJet is the most studied, most versatile, market-leading thrombectomy platform designed to enable single-session removal of thrombus. The highly effective combination of optional thrombolytic therapy with mechanical thrombectomy may help reduce ICU and total hospital stays due to:
- Faster restoration of flow
- Less lytic
- Shorter treatment time13
AngioJet treats the full range of thrombus:
| Arterial | For acute limb ischemia, AngioJet quickly restores flow and may reduce the need for prolonged lytic therapy and procedure time, leading to a potential cost benefit.14 |
| Venous | Designed specifically to treat deep vein thrombosis, the AngioJet™ ZelanteDVT™ Thrombectomy Catheter paired with ClotHunter™, can increase single-session success and avoid the need for re-intervention. |
| AV access | AngioJet is designed to offer quick removal of thrombotic materials from the dialysis access conduit, potentially improving long-term patency.15 |
Advancing the treatment of cancer and other complex interventional procedures
From the best access and embolization devices to the best therapy devices, Boston Scientific is committed to interventionalists and their patients.
Unrivaled portfolio with targeted treatments
- The largest Interventional Oncology (IO) and Embolization portfolio with a robust pipeline
- TheraSphere™ - the only Y-90 therapy approved for hepatocellular carcinoma
- EmboldTM Fibered Coil, a fully retractable, .018”, highly thrombogenic15 coil built to maximize procedural flexibility and reliability
Leading-edge clinical research
- ~$60M per year in R&D and clinical investments specific to IO and Embolization
- 14 trials for expanded indications to treat more disease states
- 3,000 patients enrolled in clinical studies and registries16

Advancing the specialty
- 5,000+ physicians and techs trained and educated17,18
- 50 global investigator-initiated studies with support from Boston Scientific
- Therapy awareness and education programs for Interventionalists and beyond