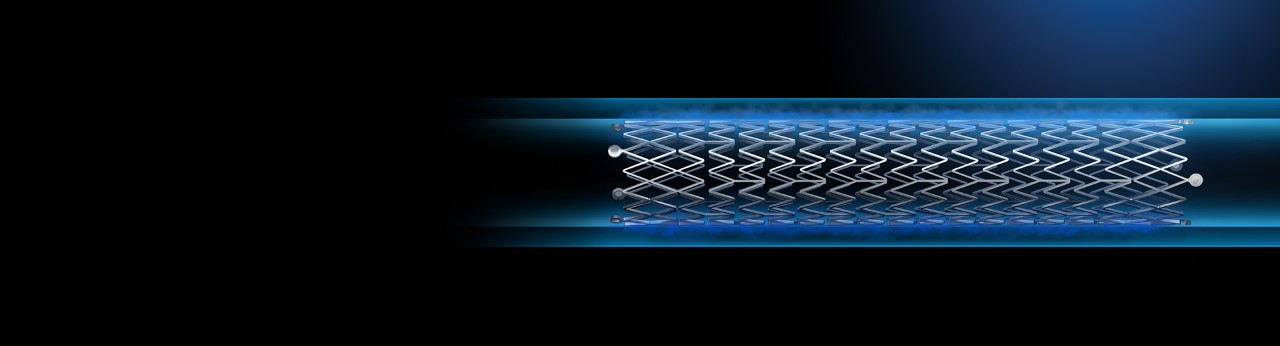
Polymer designed for controlled drug release
Eluvia DES has the lowest drug dose2 (0.167 µg/mm2) delivered by the world’s most proven polymer. Built to ensure targeted delivery to the lesion while minimizing downstream particulates and features the lowest systemic drug exposure for the patient among all peripheral paclitaxel-based technologies3.
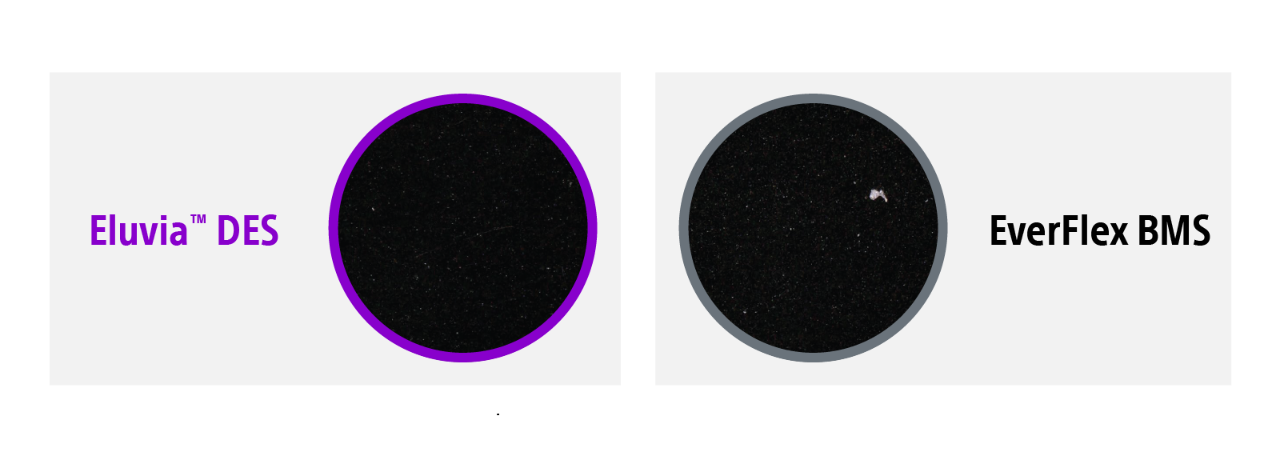
Eluvia's downstream impact
Eluvia’s polymer minimizes downstream particulates and showed similar particulate loss compared to a bare metal stent4.
Eluvia size matrix
Now available in 150mm length. Ask your sales representative about Eluvia 150.
| Stent Length | ||||||
| 40 mm | 60 mm | 80 mm | 100 mm | 120 mm | 150 mm | |
| 6 mm | 6F | 6F | 6F | 6F | 6F | 6F |
| 7 mm | 6F | 6F | 6F | 6F | 6F | 6F |
Triaxial delivery system
Built for more precise and predictable stent placement.