All Access.
Ranger Drug-Coated Balloon now available in 200cm monorail platform.
Delivering Better Outcomes: Dr. Sayfo on Ranger's Impact in PAD Care
Dr. Sam Sayfo, an interventional cardiologist, shares his experience with the Ranger DCB and why he trusts the clinical evidence backing this technology.
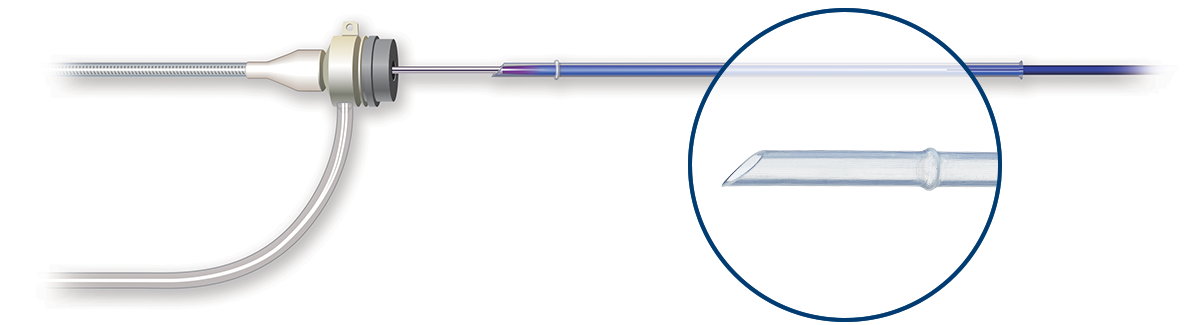
Low Tip Entry and Crossing Profile of any SFA DCB
Ranger DCB's low profile and tapered tip2 enables a smooth delivery with excellent trackability and navigation through alternative access3 sites.

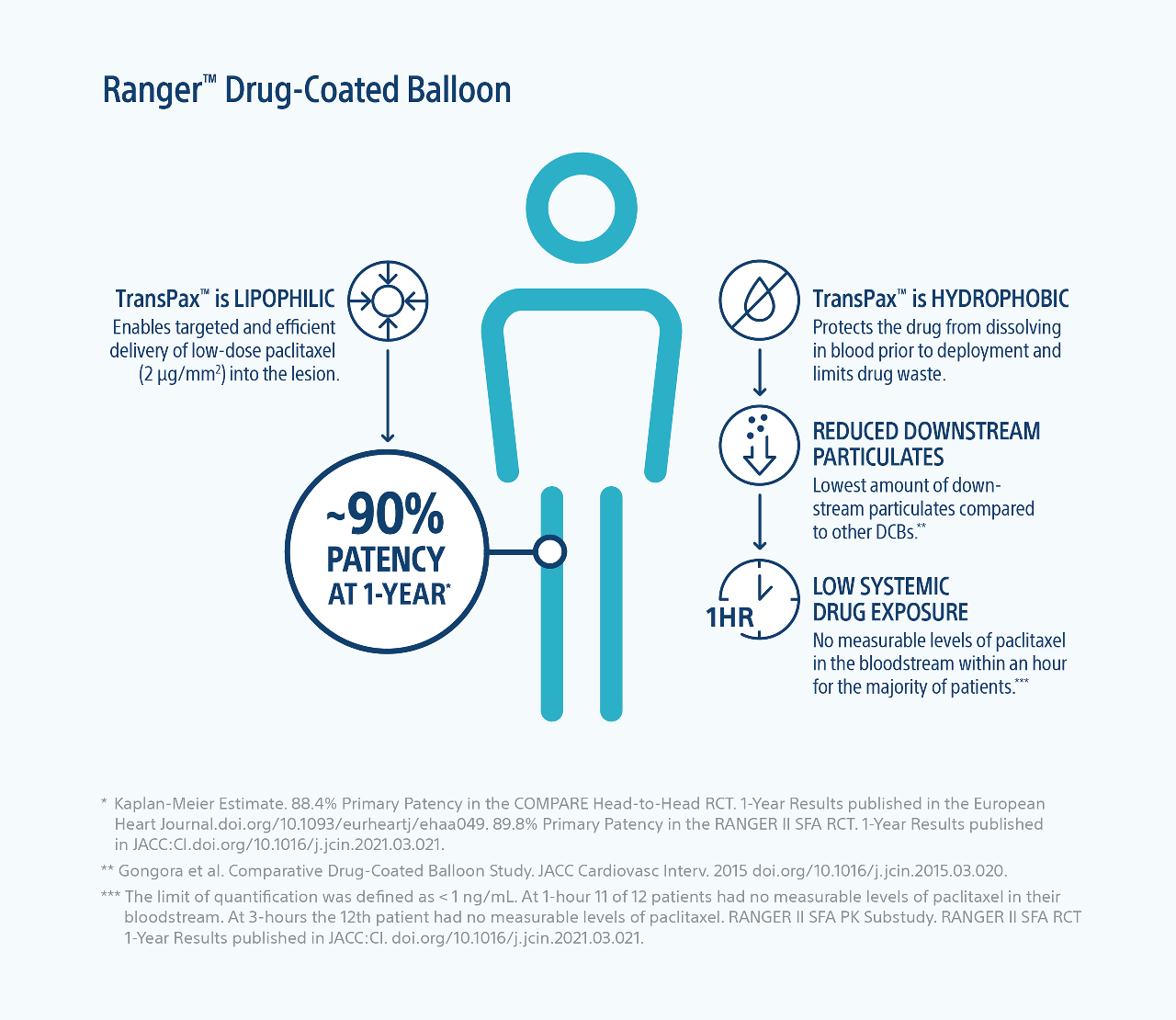
Efficient Drug Transfer
TransPax™ (citrate ester + low dose paclitaxel) is a next-generation coating that enables highly efficient drug transfer into the lesion.
Ranger DCB Product Details
Ranger DCB has a comprehensive matrix and is compatible with various access sites including pedal access3.
| Ranger matrix | 40 mm | 60 mm | 80 mm | 100 mm | 120 mm | 150 mm | 200 mm |
| 4 mm | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F |
| 5 mm | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F |
| 6 mm | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F |
| 7 mm | 6 F | 6 F | 6 F | 6 F | 6 F | 6 F | 6 F |
Over-the wire catheter with working lengths of 80cm, 90cm, 135cm, 150cm, and NEW Monorail 200cm working length and 35cm exchange length
Proprietary Loading Tool
Serves as the balloon and drug protector to help prevent drug loss during insertion and limit a physician’s exposure to the drug.