EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator
ATLAS Randomized Controlled Trial: S-ICD Superior to TV-ICD1
Study overview
The ATLAS Trial is an investigator-sponsored research study (ISR) initiated, designed and led by Population Health Research Institute (PHRI), Jeff S. Healey MD, MSc, FHRS, and the ATLAS Steering Committee. It is the first prospective randomized controlled trial where the primary objective was to evaluate lead-related complication rates between the S-ICD and single chamber TV-ICD devices at six months after implant.
Hypothesis
The trial hypothesis is that S-ICD is superior to TV-ICD with respect to serious lead-related complications*, including:
- Moderate-severe or severe tricuspid regurgitation
- Hemothorax or pneumothorax
- Cardiac perforation, tamponade, or pericardial effusion or pericarditis
- Ipsilateral upper extremity deep vein thrombosis
- Lead dislodgement or loss of sensing or pacing requiring revision
Sample Size and Timing
The trial randomized 503 patients, who passed electrocardiographic screening, from clinical centers across Canada between February 2017 and July 2021.
Primary Outcomes
Patients in the S-ICD group showed significantly fewer serious lead-related complications* than patients implanted with a single-chamber TV-ICD.


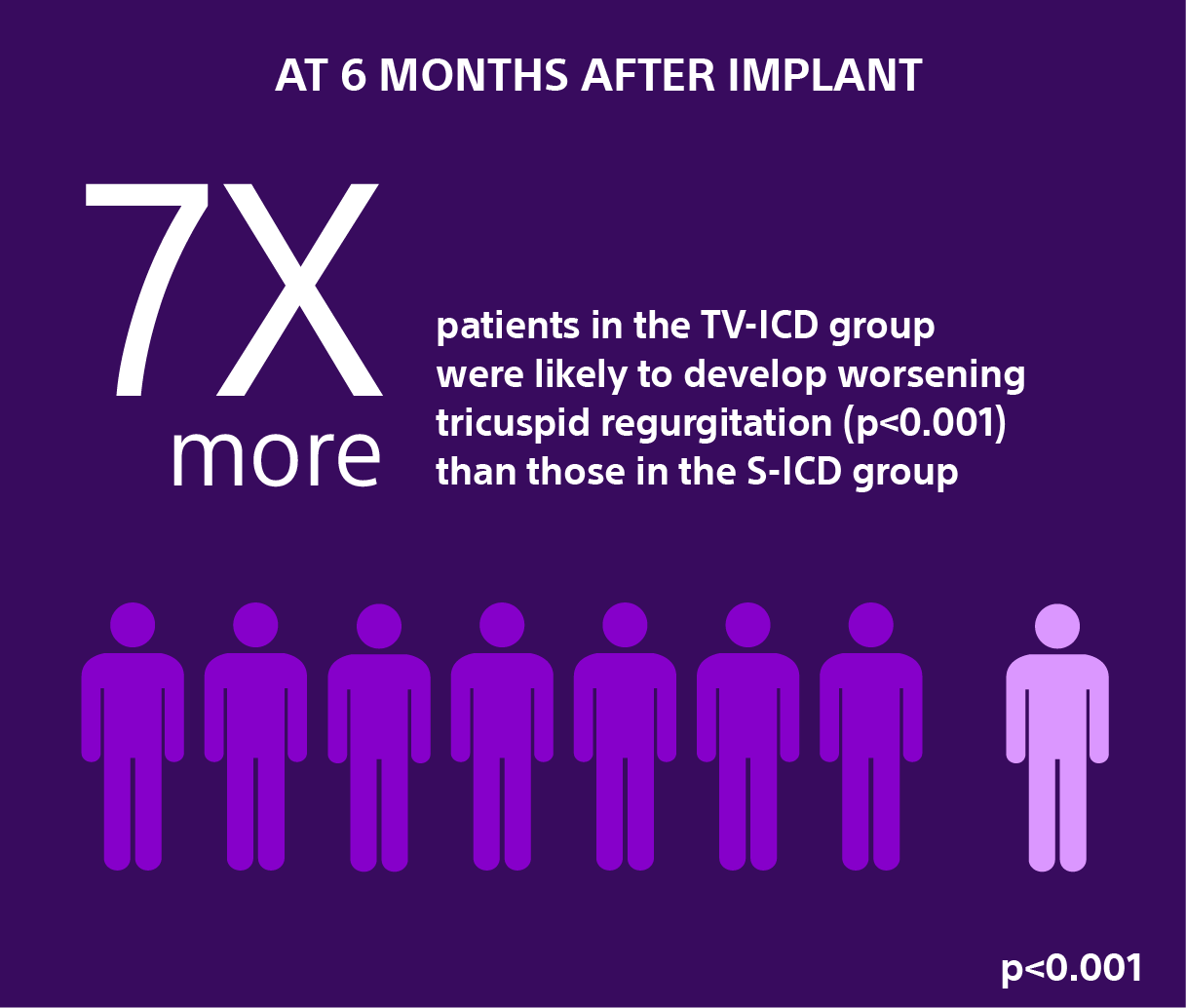
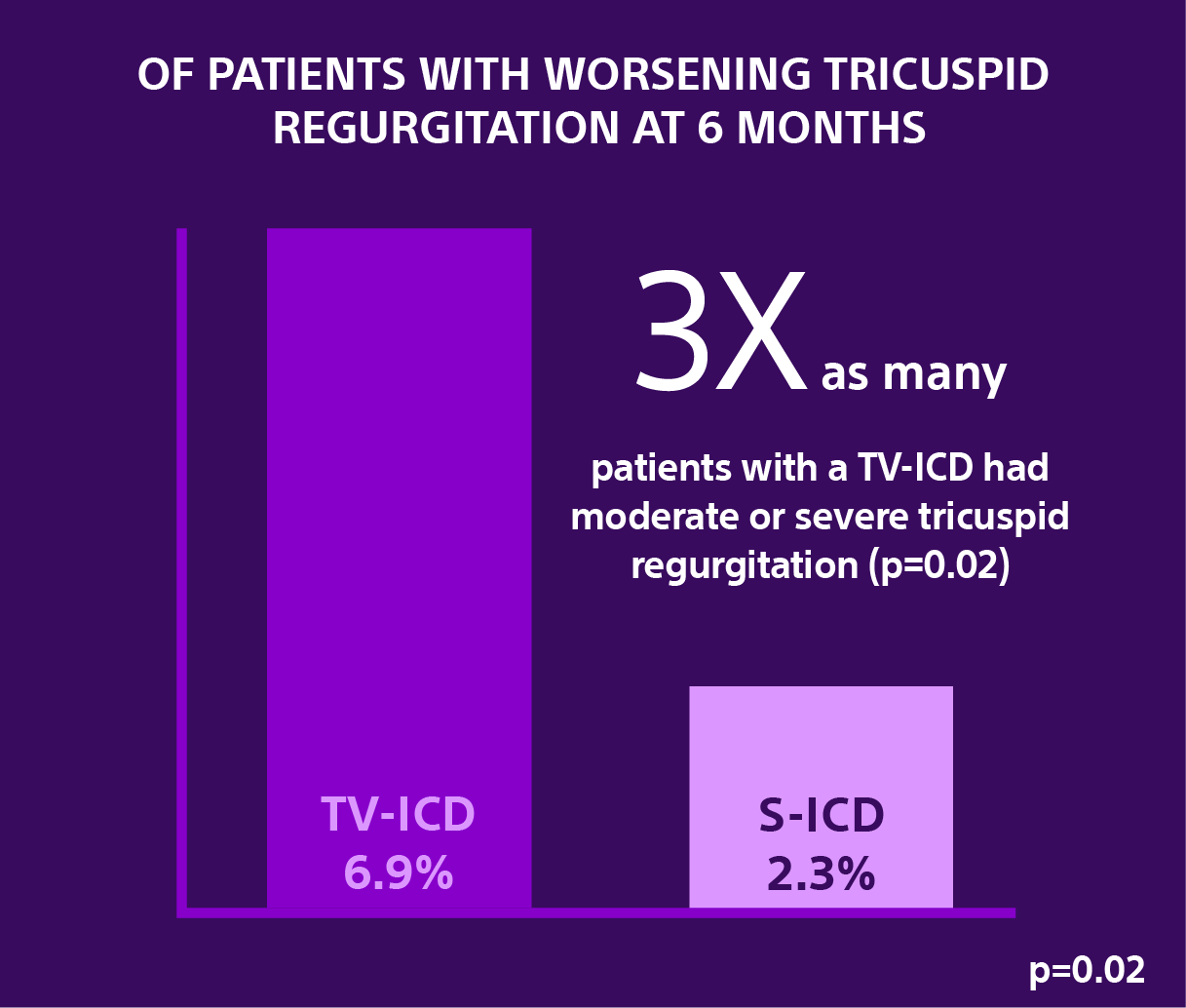
Secondary Analysis: Effects of Implantable Cardioverter-Defibrillator Leads on the Tricuspid Valve and Right Ventricle: A Randomized Comparison of Transvenous versus Subcutaneous Leads.2
Data from a secondary analysis of the investigator-sponsored research ATLAS trial evaluated in nearly 450 patients the severity of Tricuspid Regurgitation (TR) at six months following the implantation of a transvenous implantable cardioverter-defibrillator (TV-ICD) versus the EMBLEM S-ICD.