EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator
MODULAR ATP trial: Safety and efficacy of the mCRM™ system
APPRAISE ATP trial: ATP plus shock vs. shock-only
ATLAS Trial: Lead-related complication rates
PRAETORIAN: S-ICD vs. TV-ICD
Analysis of the UNTOUCHED study
Hypothesis
The incidence of inappropriate shocks in primary prevention, LVEF ≤ 35% patients will be non-inferior to the rate in transvenous ICD patients with similar programming observed in MADIT-RIT Arms B and C.
Study Design
- Follow-up for 18 months
- Device programming with a conditional zone of 200 bpm and a shock zone of 250 bpm
- Primary endpoint of inappropriate shock-free rate at 18 months
- Secondary endpoints of all cause shock-free rate at 18 months and system and procedure complications at 30 days
Key Takeaways
1. In the UNTOUCHED study a subsequent sub-analysis demonstrated that the inappropriate shock rate with SMART Pass On was 1.8% at 1 year for EMBLEM™ S-ICDs. This is the lowest reported inappropriate shock rate for S-ICD, despite a cohort with more left ventricular dysfunction and heart failure.2,3
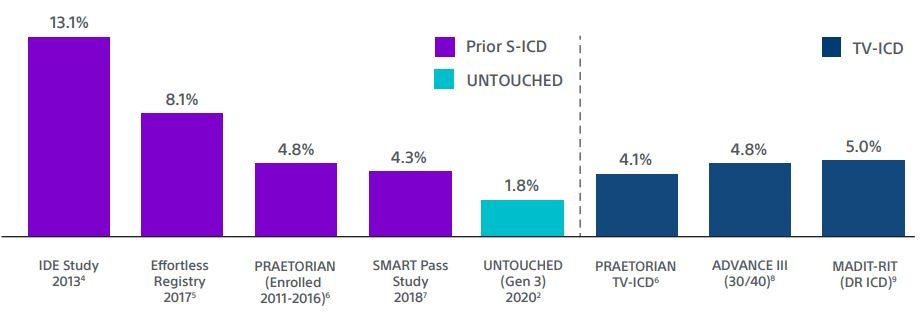
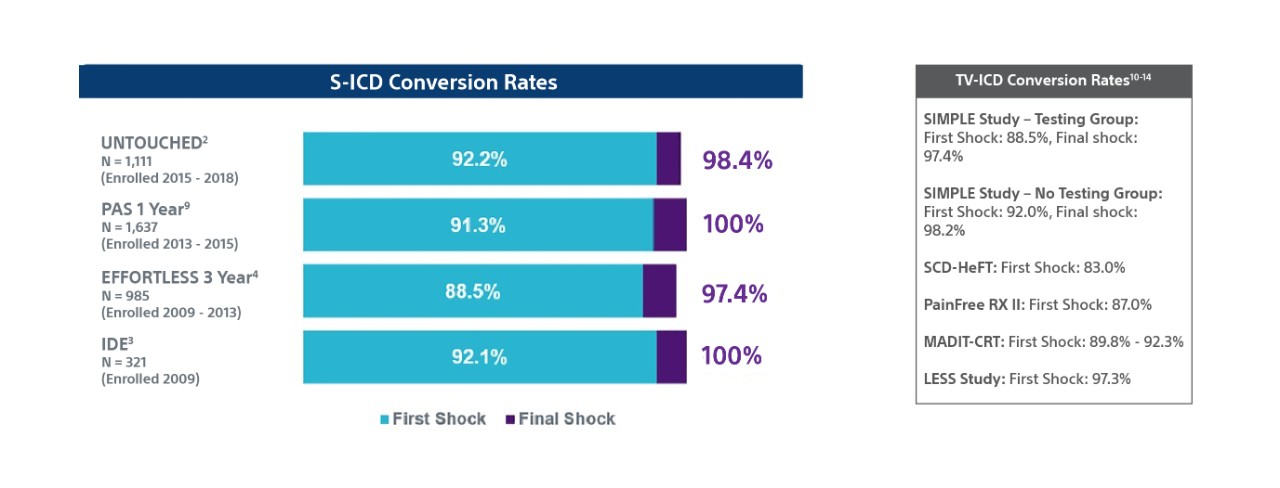
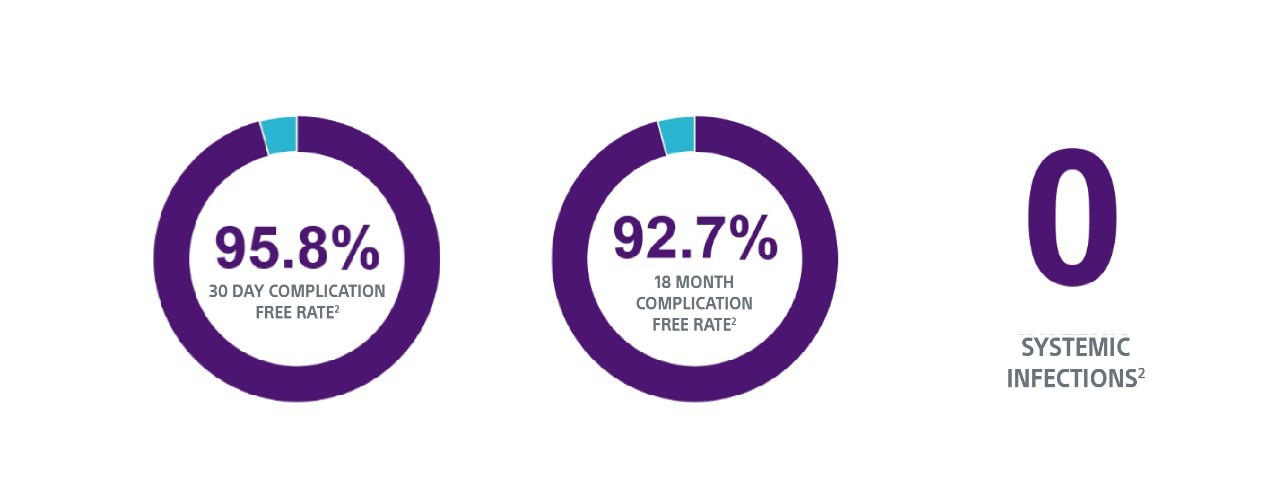
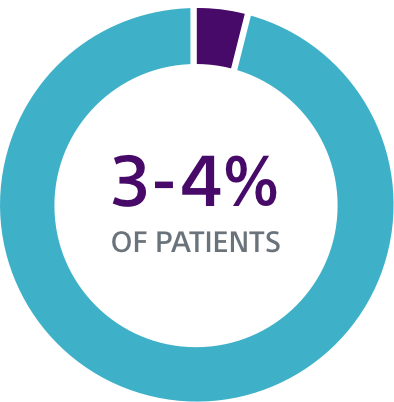
TV-ICD lead complications in the real world
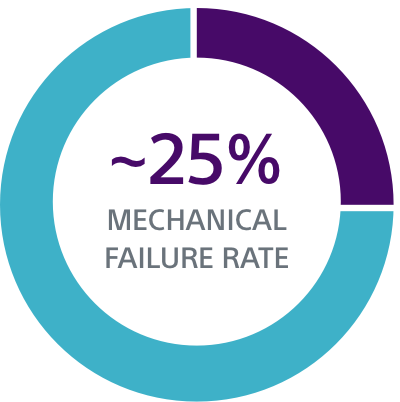
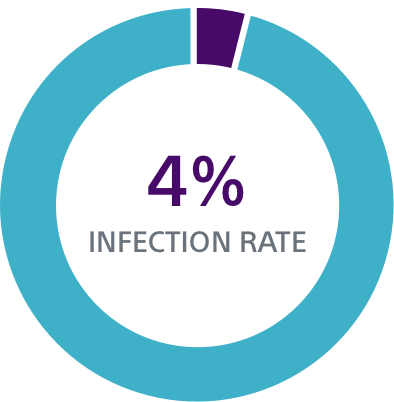
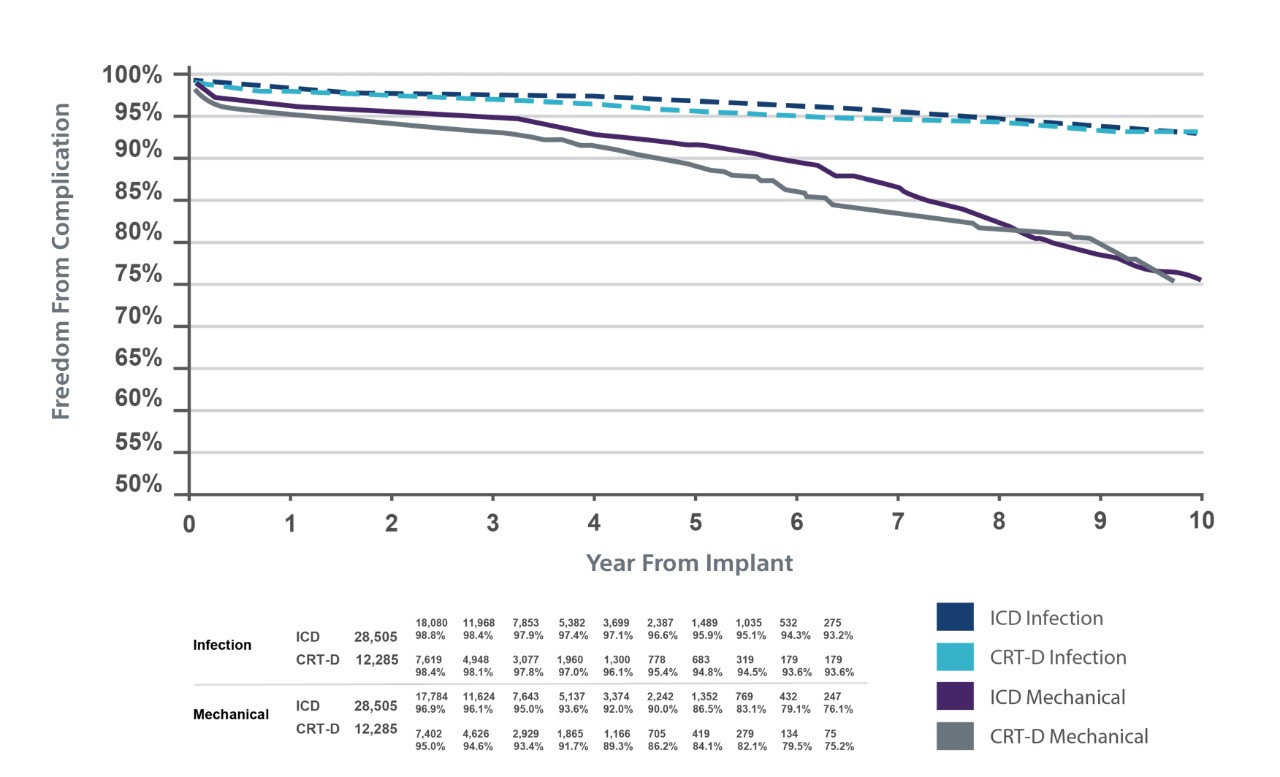
Clinical data resources
The Impact of SMART Pass on IAS
Training & education
Explore continuing education courses, best practices modules and other training and resources for S-ICD.
Why S-ICD?
See how S-ICD helps protect patients at risk for sudden cardiac death while also eliminating the risk of TV-ICD lead complications.
