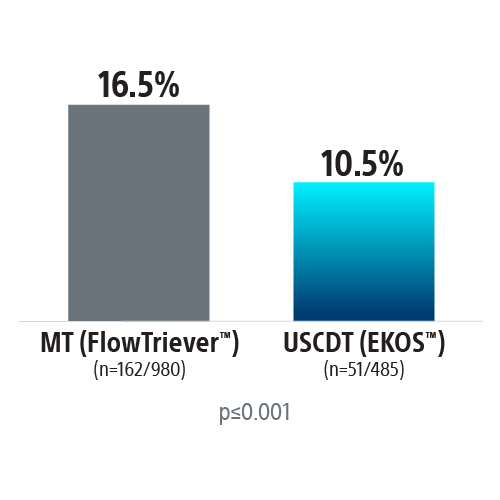
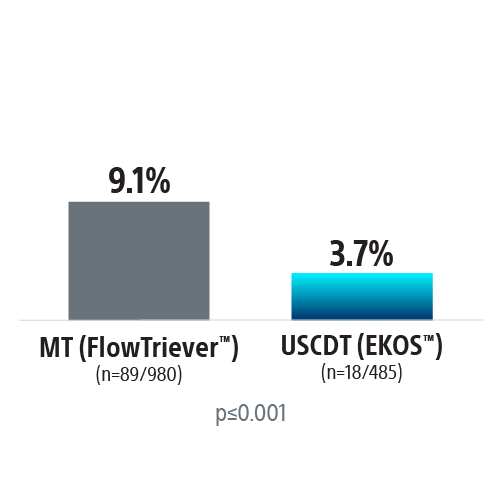
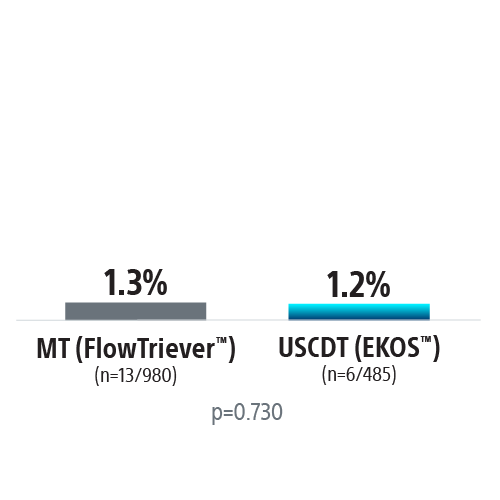
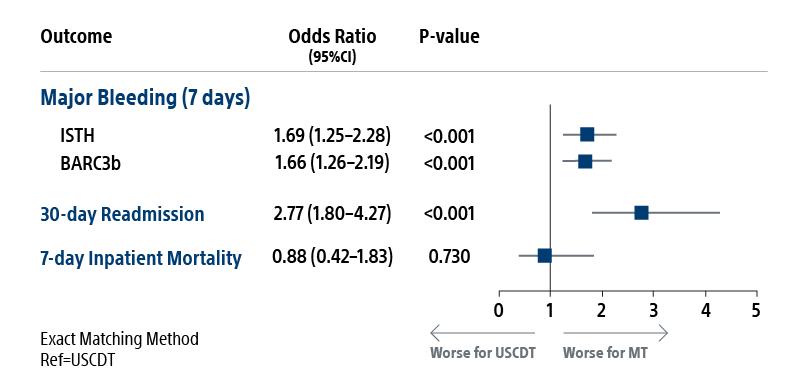
REAL-PE II builds on the REAL-PE analysis by investigating the safety of ultrasound-assisted catheter-directed thrombolysis (USCDT; i.e. the EKOS System) and mechanical thrombectomy (MT; i.e. the FlowTriever™ System, Inari Medical™) in real-world contemporary treatment of pulmonary embolism (PE), using de-identified electronic health records from Truveta™.
Methods
Propensity-score matched analysis was conducted to compare risk of major bleeding, inpatient mortality, and 30-day readmission between USCDT and MT groups. Patients were matched on demographics and medical history.
Real-PE II study patients
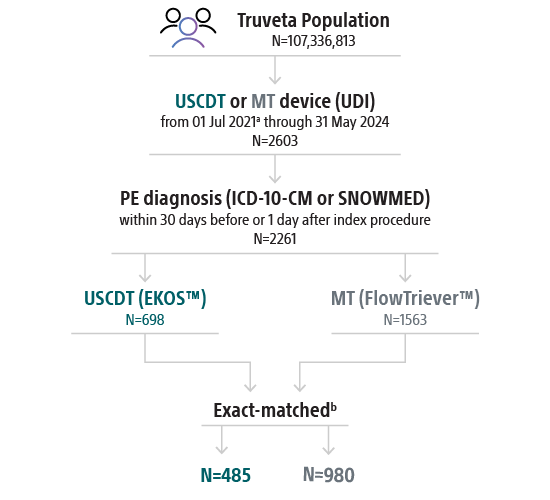
Patients
- 2261 unique PE patients before propensity-matching
- 1,465 exact matcheda patients