Boston Scientific accounts are for healthcare professionals only.
HI-PEITHO – First large-scale RCT evaluating intervention vs SOC
Trial overview
Multi-center
Prospective
Randomized
Controlled
Transatlantic
Importance
The HI-PEITHO study has been designed to address a critical gap in clinical evidence in PE by comparing the clinical benefit of intervention with EKOS vs. the current standard of care – anticoagulation. This trial is the largest and first of its kind in PE. It is designed to generate the most rigorous, highest level of data, contributing to the body of evidence for the treatment and outcomes in acute, intermediate-high risk PE. In addition, HI-PEITHO aims to advance the understanding of intermediate-high risk PE and risk stratification, to better identify patients who may clinically benefit from intervention, expanding access to care for these patients.
Designed to reduce industry bias
HI-PEITHO is not your typical industry study. The unique research partnership with the PERT Consortium and the University of Mainz aims to reduce industry bias.
Unlike other clinical studies in PE, HI-PEITHO includes objective, clinically significant measures and bailout criteria (not subjective measures such as ICU time).
Summary
HI-PEITHO is a multi-center, prospective, randomized, controlled trial in the U.S. and Europe that will compare the outcomes of ultrasound-facilitated, catheter-directed, thrombolysis plus anticoagulation vs. anticoagulation alone for the treatment of acute, intermediate-high risk pulmonary embolism (PE).
Objective
EKOS™ Endovascular System + Anticoagulation vs. Anticoagulation Alone
Evaluate if treatment with EKOS is associated with a significant reduction in the acute composite outcome of the measures below compared to anticoagulation alone –
- PE-Related Death
- Cardiorespiratory decompensation or collapse, and
- Non-fatal symptomatic and objectively confirmed recurrence of PE
Trial methodology
Patients
- Enrollment: 544 participants
- Acute intermediate high-risk pulmonary embolism
- RV/LV > 1.0, elevated troponin, & risk of early death/hemodynamic collapse
Trial design
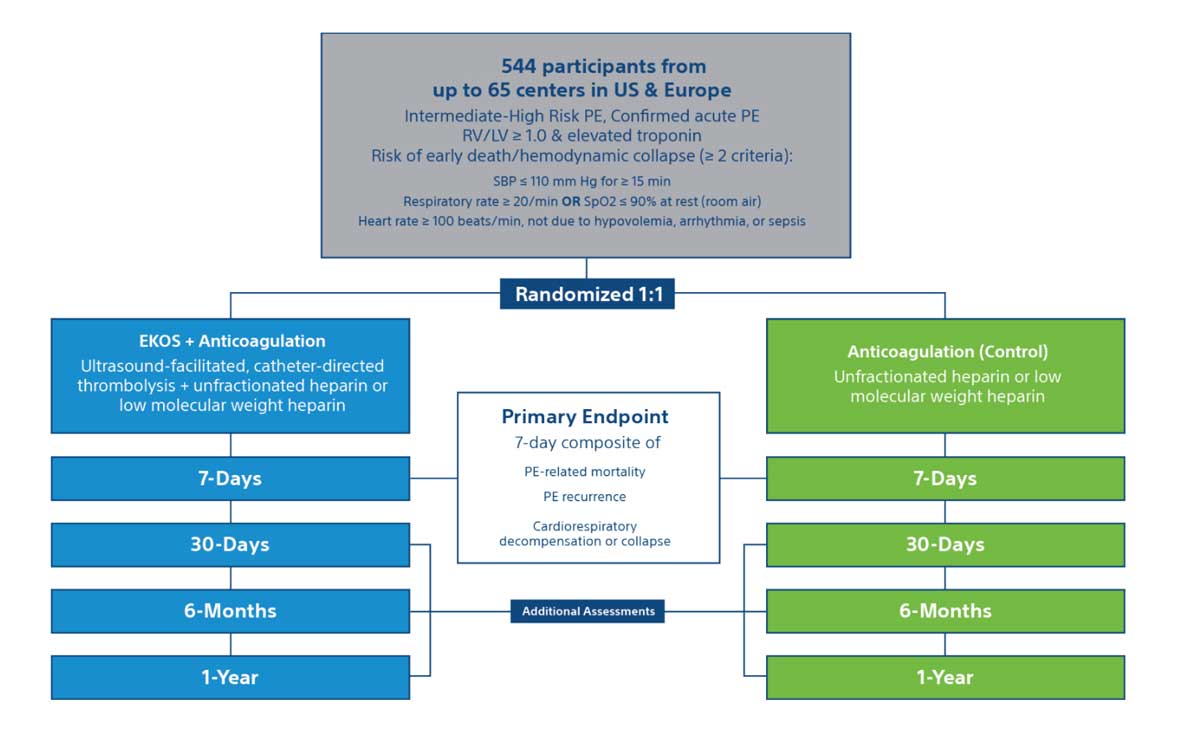
Trial end points & assessments
Independent
review
Long term
Blinded outcome adjudication
End Points
7-day composite of –
- PE-related mortality
- PE recurrence (non-fatal symptomatic and objectively confirmed)
- Cardiorespiratory decompensation or collapse
Cardiorespiratory decompensation or collapse, defined as at least one of the following:
- Cardiac arrest or need for CPR
- Signs of shock: new onset arterial hypotension with end-organ hypoperfusion
- ECMO placement
- Intubation or noninvasive mechanical ventilation
- National Early Warning Score (NEWS) of 9 or higher
Long Term Follow-Up
Additional follow-ups at 30-days, 6 months, and 1-Year
National Early Warning Score (NEWS)
Earlier warning
Standardizing
assessment
Objectifying patient experience

Reproduced from: Royal College of Physicians. National Early Warning Score (NEWS): Standardising the assessment of acute-illness severity in the NHS. Report of a working party. London: RCP, 2012
The NEWS is an early warning system score for clinical deterioration in hospitalized patients – it standardizes the assessment of acute-illness severity.
The score aims to objectify how the patient is doing at the bedside.
The NEWS is based on an aggregate scoring system based on physiological measurements – respiration rate, oxygen saturations, supplemental oxygen need, temperature, systolic blood pressure, heart rate, and level of consciousness.
Key additional assessments
Functional & QOL
measures
Two bleeding
measurements
Health economic
assessment
- Individual primary outcome components
- GUSTO major (moderate and severe) bleeding within 7 days
- International Society on Thrombosis and Haemostasis (ISTH) major bleeding
- Ischemic or hemorrhagic stroke within 7 days and 30 days
- All-cause mortality
- Symptomatic PE recurrence within 30 days and 6 months
- Change from baseline in RV dysfunction on echocardiography at 6 months
- Chronic thromboembolic pulmonary hypertension (CTEPH) diagnosis within 12 months
- Health economic assessments
- Functional status and quality of life measures
- Cardiopulmonary Exercise Testing (CPET) at select sites



