Study objective
To understand the impact of the EKOS Endovascular System low-dose, short treatment duration OPTALYSE PE study on various ultrasound-accelerated thrombolysis (USAT) protocols being used as the standard of care in the treatment of acute pulmonary embolism and associated long-term outcomes including quality of life outcomes.
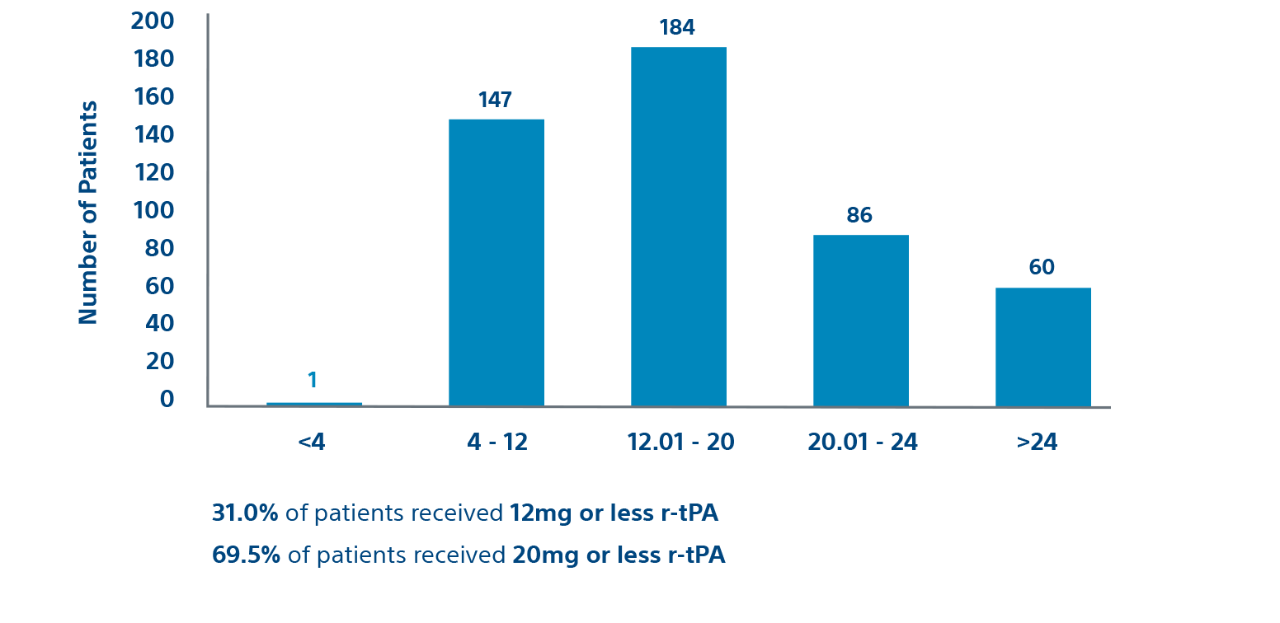
489
489 patients in the prospective cohort
O ICH*
Zero intracerebral hemorrhagic events
23%
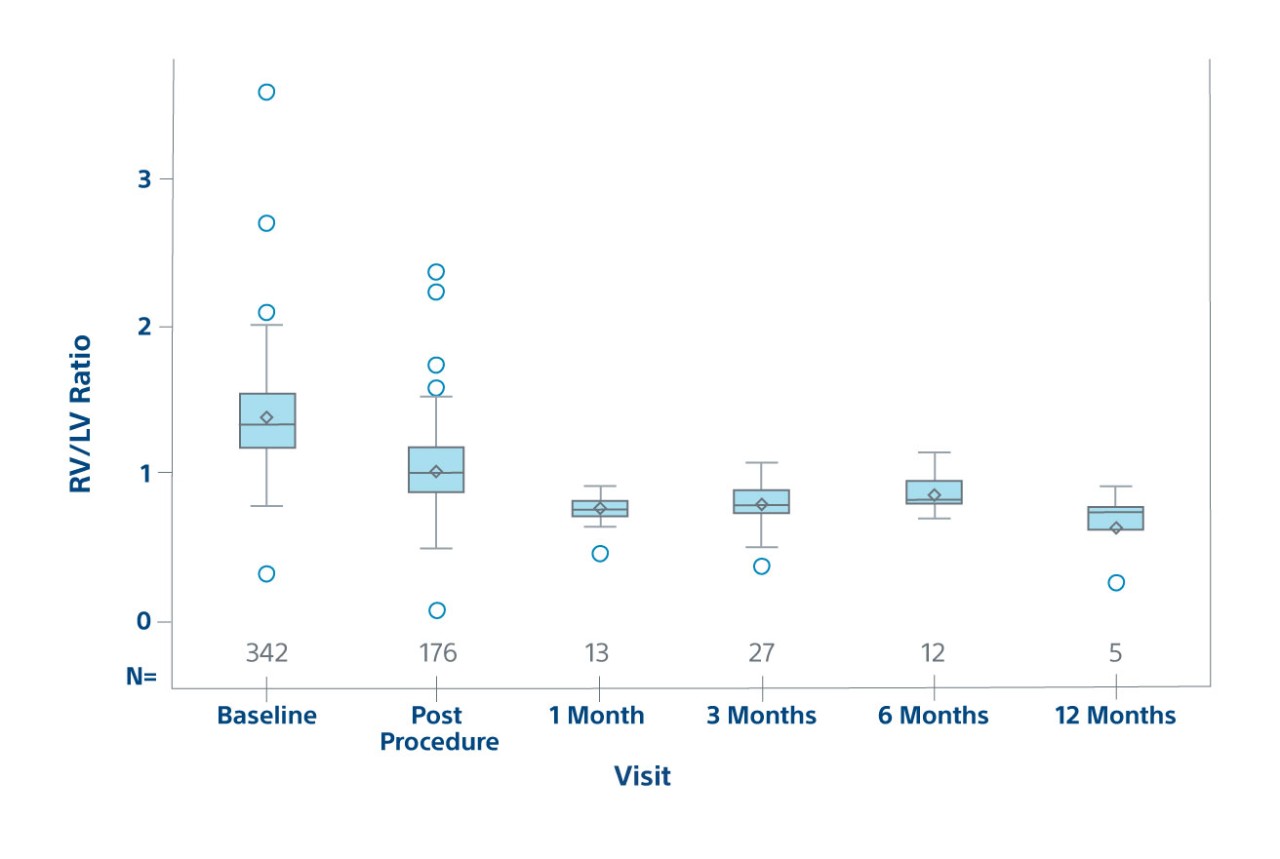
23% RV/LV reduction post-procedure
Results
Safety
Major bleeding at 72 hours | 8 (1.6) |
| Gastrointestinal hemorrhage | 2 (0.4) |
| Head laceration | 1 (0.2) |
| Subdural hematoma (pre-existing) | 1 (0.2) |
| Vascular access site hematoma | 4 (0.8) |
Recurrent VTE Within 30 Days | |
| Pulmonary Embolism | 2 (0.4) |
* 1 preexisting subdural hemorrhage (SDH)
Procedural
Prospective cohort
Efficacy
RV/LV Ratio by Visit on ECHO
Efficacy population in prospective patients
Evidence
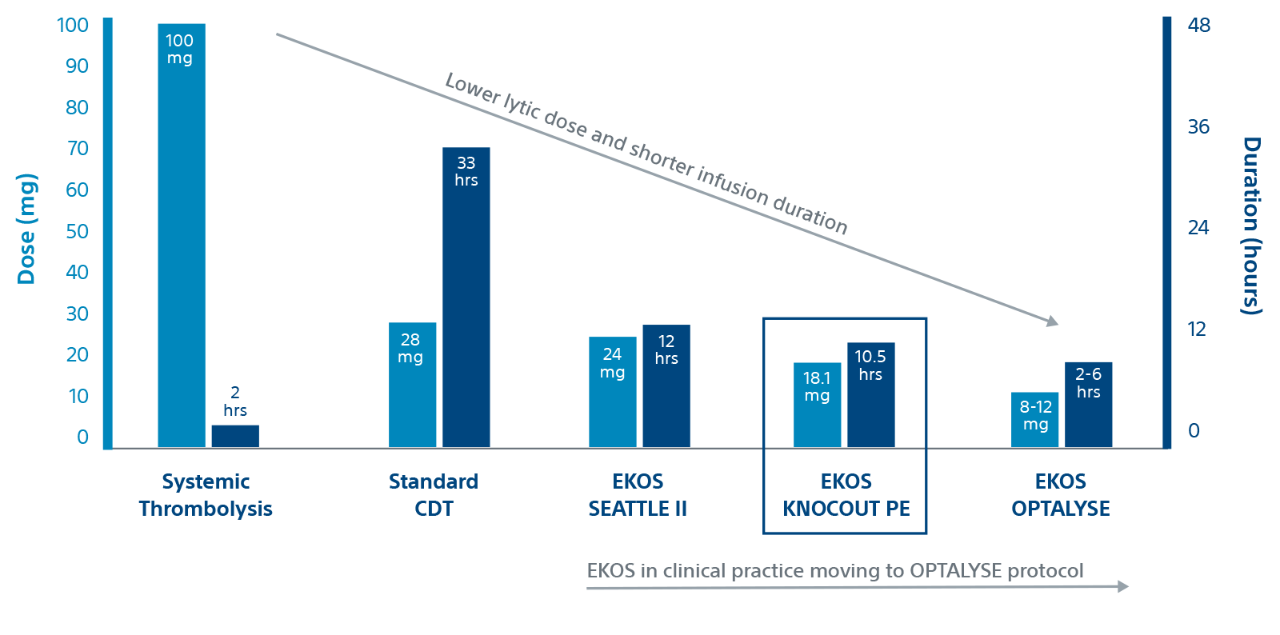
KNOCOUT shows that contemporary clinical practices are moving to low-dose, short duration OPTALYSE protocols. It adds to the growing evidence that EKOS is effective at treating intermediate-risk and high-risk PE with lower lytic doses and shorter infusion durations compared to other thrombolytic therapies.
Duration and dose by therapy