EKOS™ Endovascular System
Higher-risk Pulmonary Embolism Thrombolysis Study
Patients now enrolling!
“We are pleased to see the official start to the HI-PEITHO trial with the first patient enrollment. HI-PEITHO is the largest, most rigorous trial in the PE space intended to address critical gaps in PE clinical evidence. This trial reflects Boston Scientific’s investment and commitment to providing the highest level of scientific evidence to ultimately help guide clinical practice.”
Key Resources
HI-PEITHO – The Higher-Risk Pulmonary Embolism Thrombolysis Study
Hear from the Principal Investigators

Partnerships
Trial Overview
Importance
Summary

Objective
EKOS™ Endovascular System + Anticoagulation vs. Anticoagulation Alone
Evaluate if treatment with EKOS is associated with a significant reduction in the acute composite outcome of the measures below compared to anticoagulation alone –
- PE-Related Death
- Cardiorespiratory decompensation or collapse, and
- Non-fatal symptomatic and objectively confirmed recurrence of PE
Trial Methodology
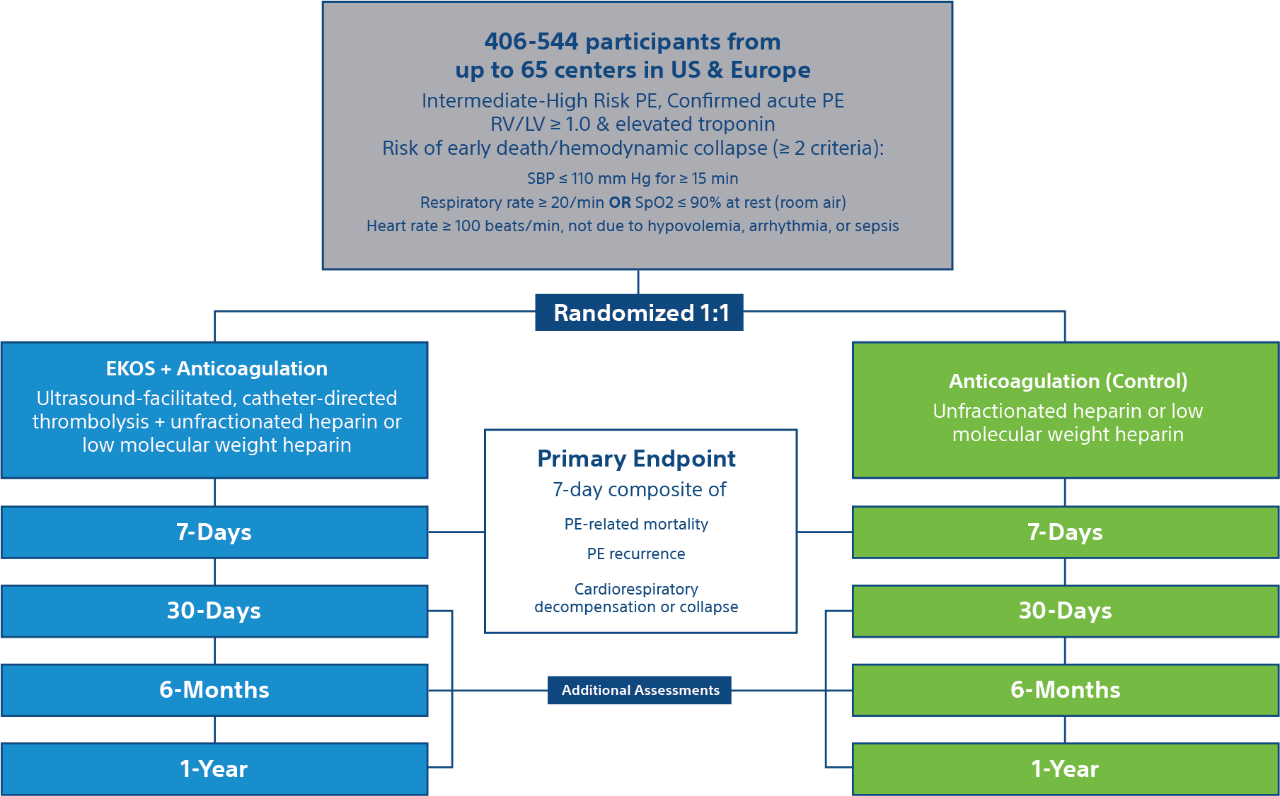
Patients
- Estimated enrollment: 406-544 participants
- Acute intermediate high-risk pulmonary embolism
- RV/LV > 1.0, elevated troponin, & risk of early death/hemodynamic collapse
Trial Design
Trial End Points & Assessments

End Points
7-day composite of –
- PE-related mortality
- PE recurrence (non-fatal symptomatic and objectively confirmed)
- Cardiorespiratory decompensation or collapse
Cardiorespiratory decompensation or collapse, defined as at least one of the following:
- Cardiac arrest or need for CPR
- Signs of shock: new onset arterial hypotension with end-organ hypoperfusion
- ECMO placement
- Intubation or noninvasive mechanical ventilation
- National Early Warning Score (NEWS) of 9 or higher
Long Term Follow-Up
Additional follow-ups at 30-days, 6 months, and 1-Year
National Early Warning Score (NEWS)

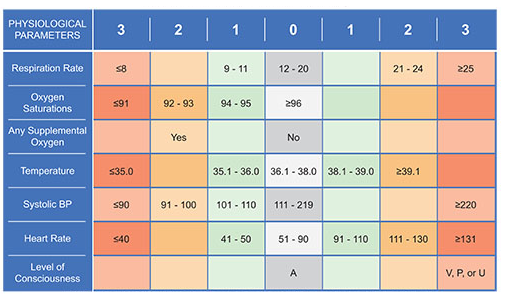
The NEWS is an early warning system score for clinical deterioration in hospitalized patients – it standardizes the assessment of acute-illness severity.
The score aims to objectify how the patient is doing at the bedside.
The NEWS is based on an aggregate scoring system based on physiological measurements – respiration rate, oxygen saturations, supplemental oxygen need, temperature, systolic blood pressure, heart rate, and level of consciousness.
Key Additional Assessments

- Individual primary outcome components
- GUSTO major (moderate and severe) bleeding within 7 days
- International Society on Thrombosis and Haemostasis (ISTH) major bleeding
- Ischemic or hemorrhagic stroke within 7 days and 30 days
- All-cause mortality
- Symptomatic PE recurrence within 30 days and 6 months
- Change from baseline in RV dysfunction on echocardiography at 6 months
- Chronic thromboembolic pulmonary hypertension (CTEPH) diagnosis within 12 months
- Health economic assessments
- Functional status and quality of life measures
- Cardiopulmonary Exercise Testing (CPET) at select sites



