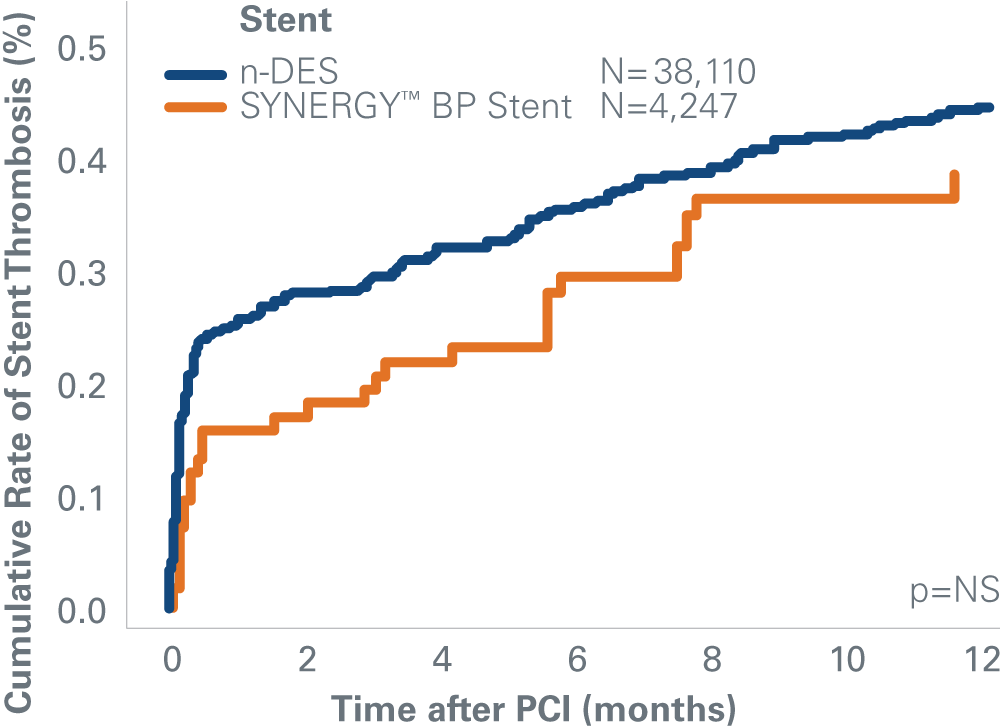
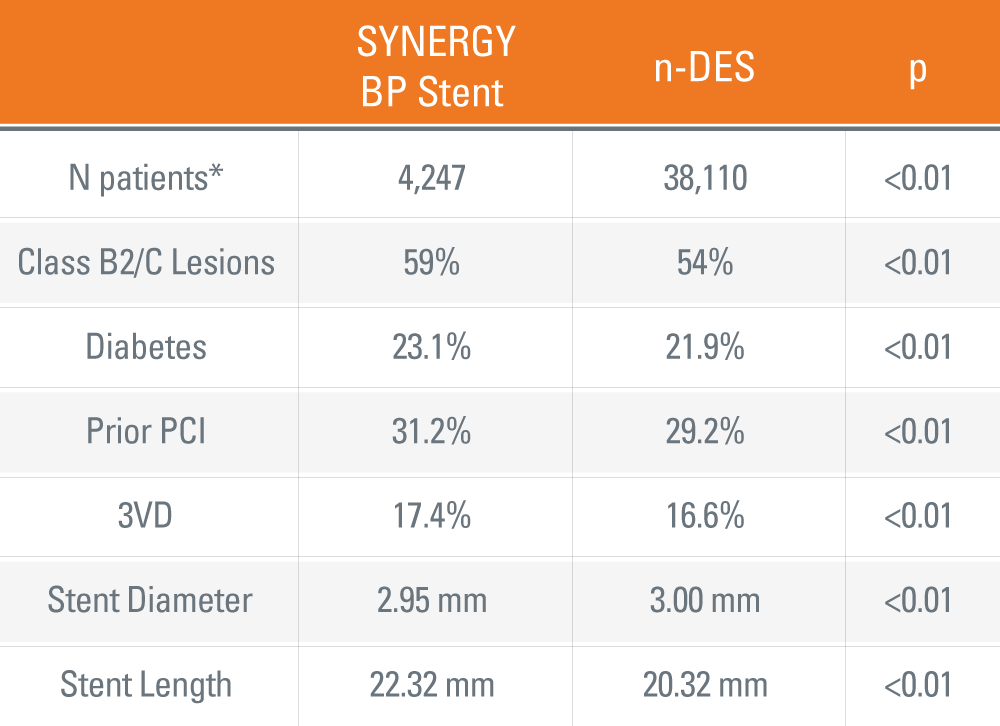
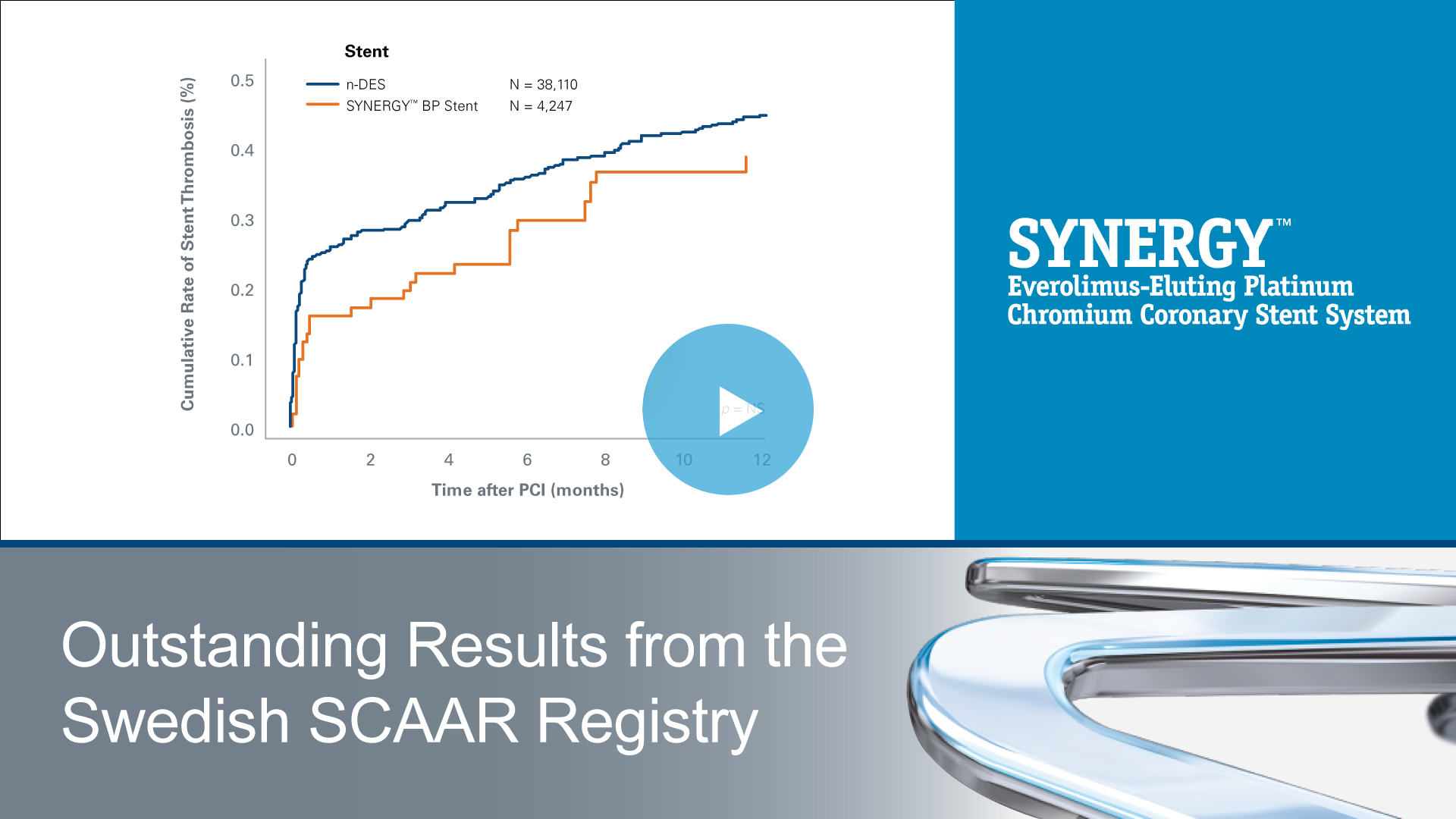
SYNERGY™
Everolimus-Eluting Platinum Chromium Coronary Stent System
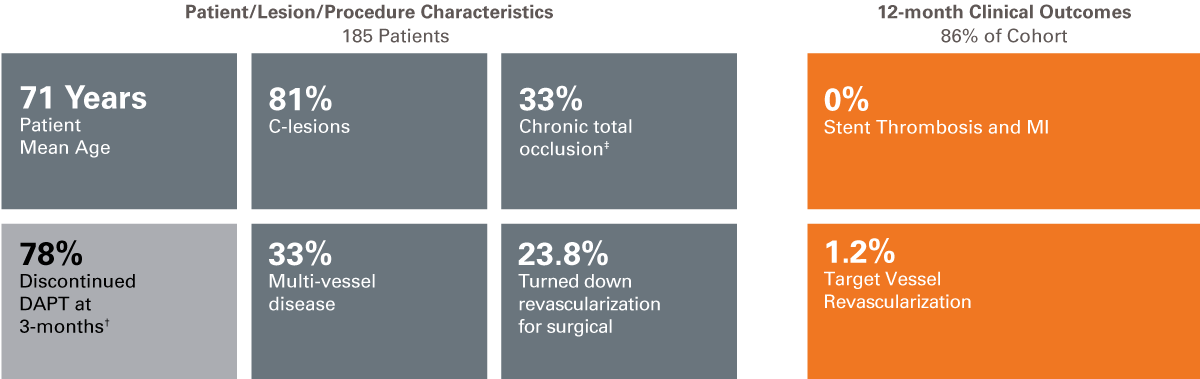
We are continuing to pursue clinical evidence of patients in real-world studies through our Investigator Sponsored Research program. Learn more about our Clinical Trial Program