Boston Scientific accounts are for healthcare professionals only.
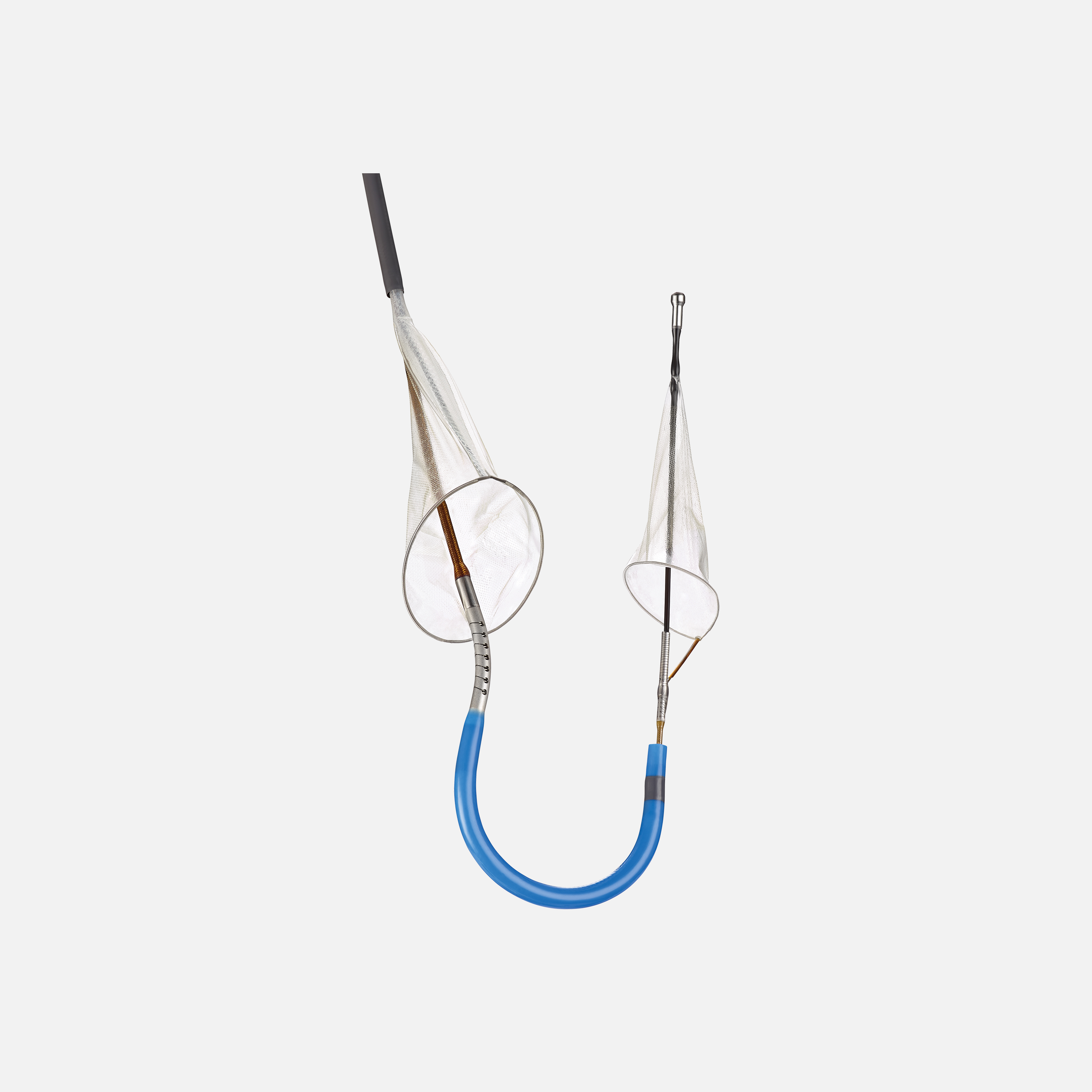
SENTINEL™ Cerebral Protection System
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical Data
- Technical Specifications
- Ordering Information
- Resources
SENTINEL™ Cerebral Protection System
Stroke Happens. PROTECTION Works.
Protected TAVR™ with SENTINEL Cerebral Protection System (CPS) gives you the power to reduce stroke.
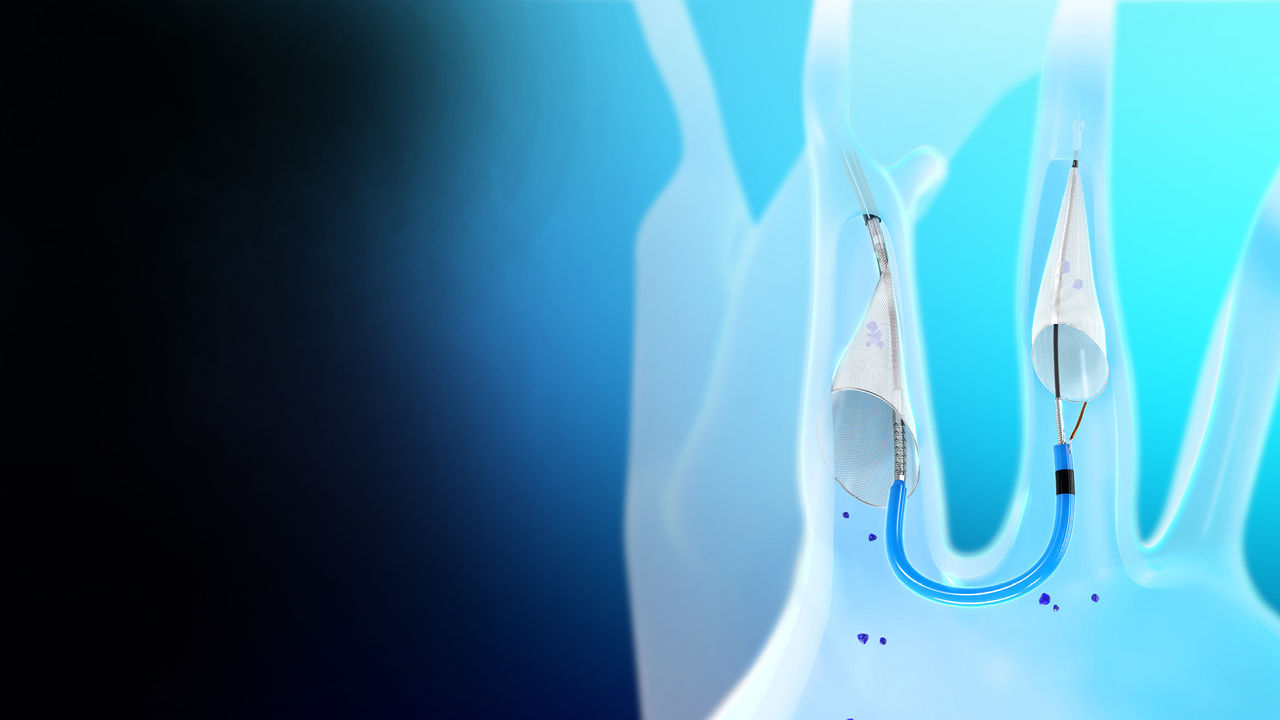
How it works
The SENTINEL CPS captures and removes debris loosened during TAVR procedures. It is delivered percutaneously immediately before a TAVR procedure and filters 90% of the blood flow to the brain.
Why choose SENTINEL™ Cerebral Protection System
As the only way to ensure that patients are protected from embolic debris, SENTINEL is safe and clinically proven to capture and remove embolic debris and reduce neurological events. Embolic debris is present in almost all TAVR procedures, and there is no way to predict when it will result in a stroke.
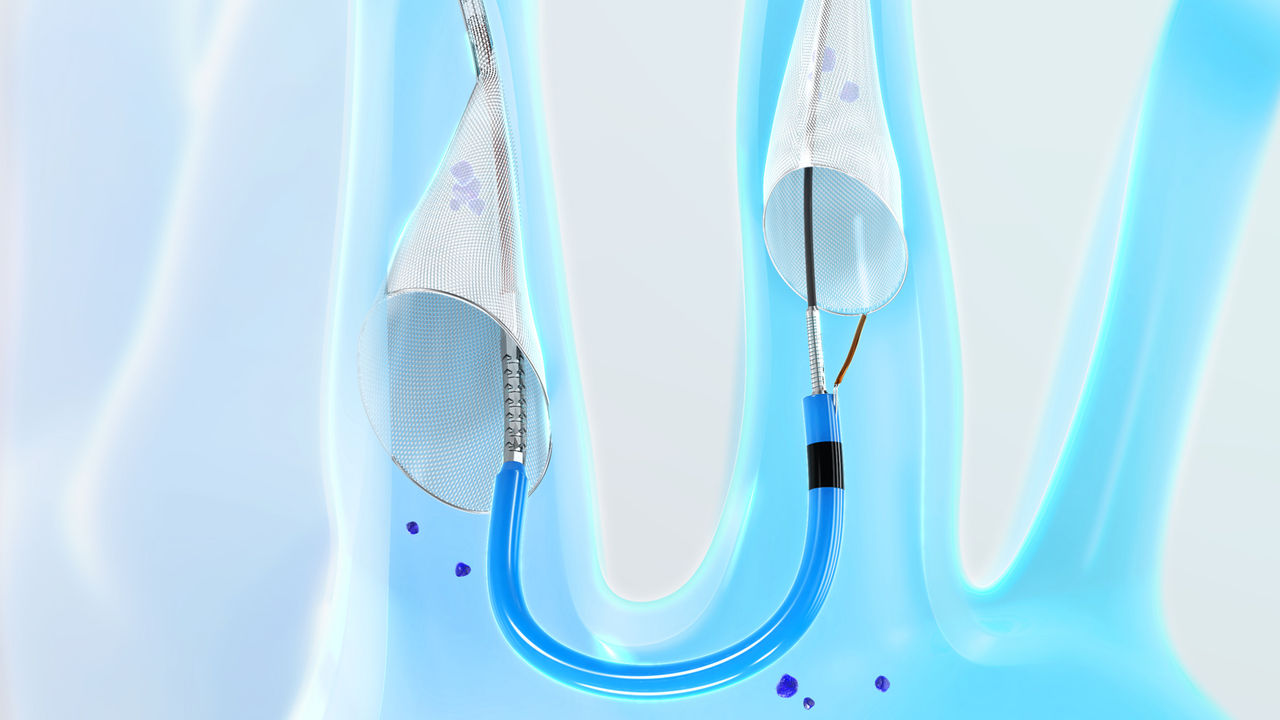
Designed for Stroke Protection
Deflecting stroke-causing embolic debris isn’t enough. SENTINEL is designed to capture and remove this debris, which was found in 99% of patients treated with SENTINEL1-4.

Backed by Trial Data
Choose with confidence, knowing SENTINEL is the most-studied cerebral embolic protection for TAVR. The first and only FDA-cleared product on the market, it has protected more than 120,000 patients from stroke worldwide.

Easy to Use
Reduce patient risk without complicating your workflow.
- SENTINEL deploys in a median time of 4 minutes5.
- Right radial access simplifies workflow for physicians.
Over time, TAVR complications have declined – stroke rates have decreased to a lesser extent. Despite clinical and technological advancements, the rate of reported perioperative stroke is holding steady at 2-4%6-11,24.
Devastating patient impact
It is impossible to predict which procedures will dislodge embolic debris, and when it will cause disabling stroke.
Stroke is the leading cause of permanent disability – the long-term effects are serious including impaired ability to communicate and restricted physical abilities. It is also a primary risk factor for future clinical stroke, dementia, cognitive decline, and mortality.
#1 cause of permanent disability
Stroke is the leading cause of long-term disability12.
#1 patient fear
Stroke is the top concern of TAVR patients, even more than death13.
78% of patients
say maintaining independence is ther top goal post-TAVR14.
The cost of stroke
For hospitals, the financial implications of all stroke include extended inpatient care, rehabilitation, and follow-up care.

Total average hospitalization cost15

Longer average length of stay15

Average hospital readmission rate15
120,000+ patients protected with SENTINEL™ Cerebral Protection System
Excellent safety
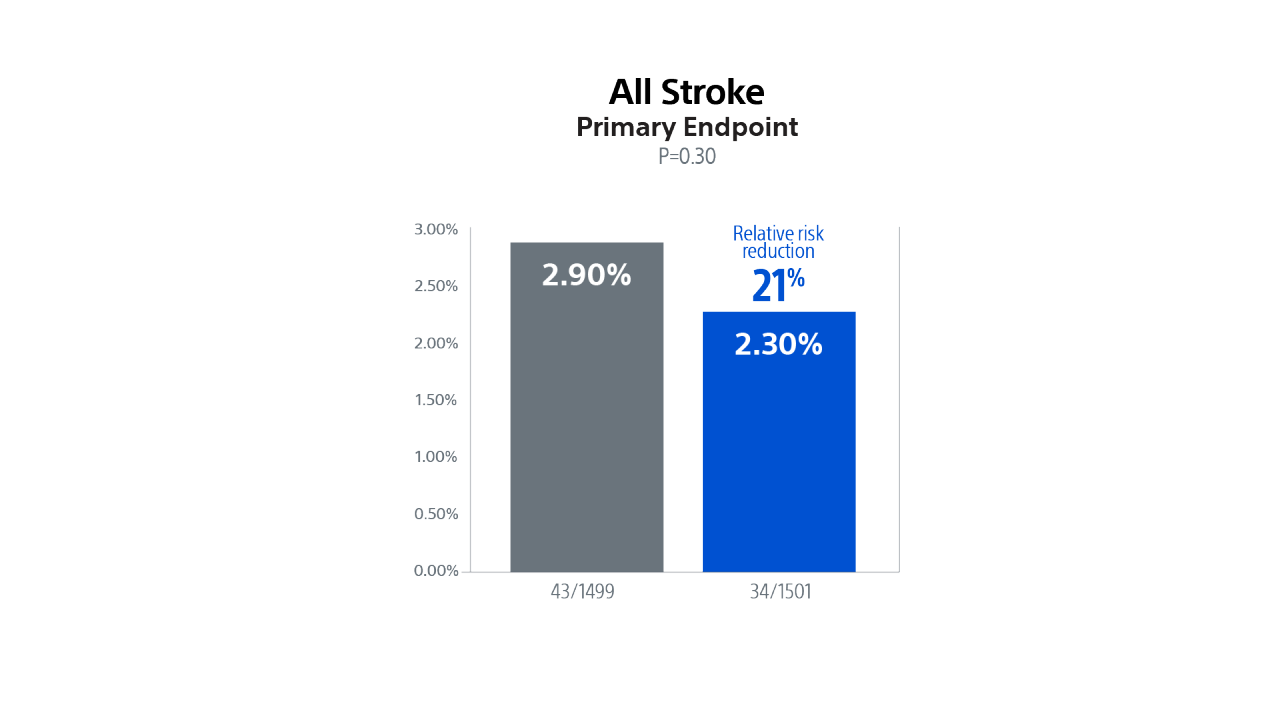
The SENTINEL CPS demonstrated excellent safety with high rates of device delivery/retrieval (94.4%) and very low rates of vascular complications (0.1%)16. Data showed a 21% relative risk reduction in all stroke and a 60% significant relative risk reduction in disabling stroke through 72-hours.
Every patient is a candidate for protection from disabling stroke
The effect of SENTINEL on disabling stroke was consistent across patients' subgroups.
Continuous improvement
Your team works tirelessly for the best possible outcomes – let us help maximize them. Providing Transcatheter Aortic Valve Replacement (TAVR)-related stroke protection is simple and life-changing.
Protect patients with minimal impact to your program
The SENTINEL CPS takes minutes to deploy and causes minimal disruption to procedural workflow17.
- 4 min median deployment time to place both filters
- 9/10 anatomies covered with one size
- Minimal interference with valve delivery
Watch the video to see the SENTINEL CPS device's dual filters seamlessly deployed.
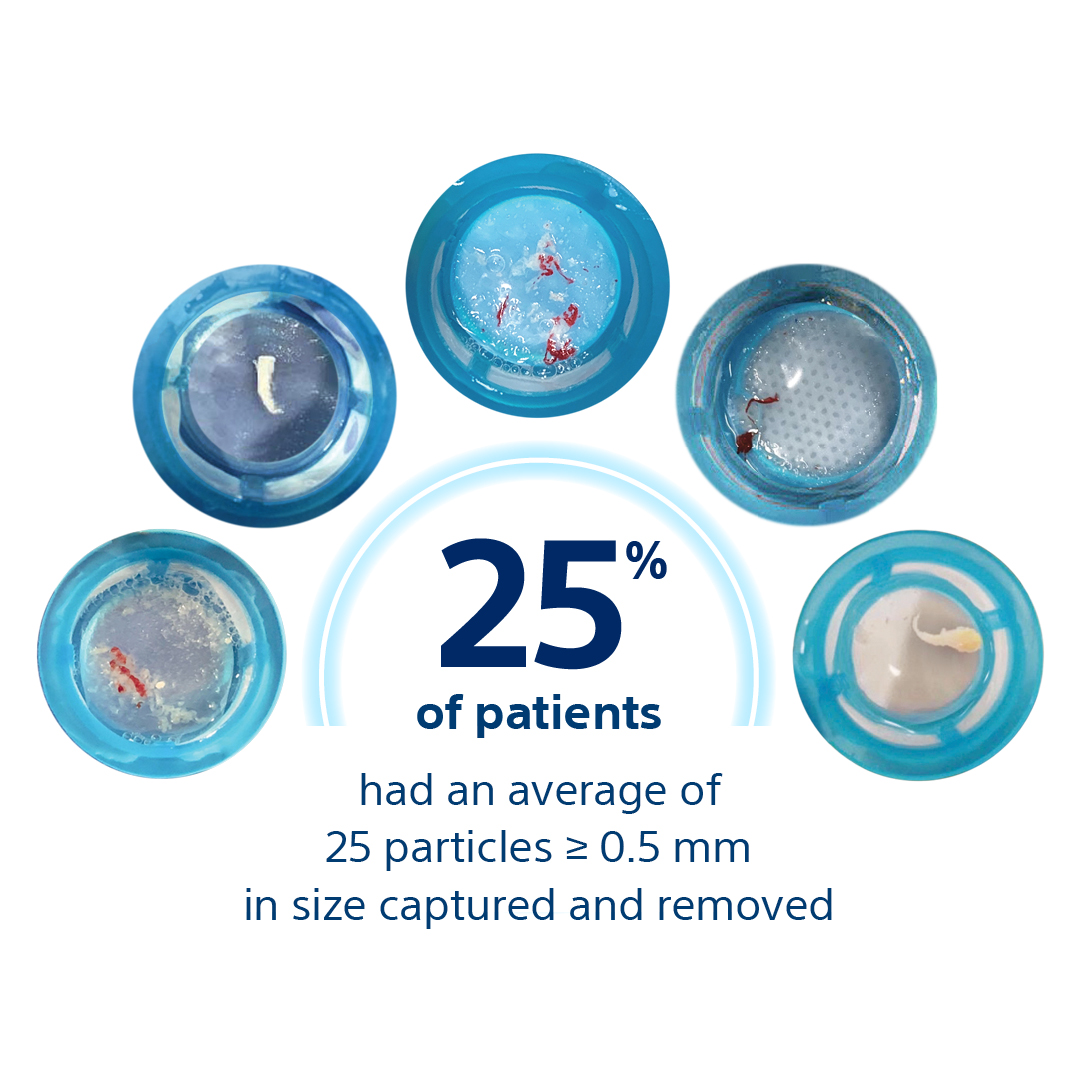
Seeing is believing
In 99% of procedures, SENTINEL was shown to capture and remove stroke-causing embolic debris such as arterial wall pieces, valve tissue, calcified and foreign material18,19.
Clinical highlights
British Heart Foundation PROTECT-TAVI Trial
Routine Cerebral Embolic Protection in Transcatheter Aortic Valve Implantation20
This landmark trial is the largest randomized TAVI trial to date, with 7,635 patients enrolled at more than 30 sites in the United Kingdom who were randomized 1:1 – patients protected with the SENTINEL Cerebral Protection System (CPS) versus no use of the SENTINEL device during TAVI.
- The primary and secondary outcomes were assessed in the modified ITT population.
- The incidence of the primary outcome of stroke at 72 hours post-TAVI or at discharge (if sooner) was comparable among both groups.
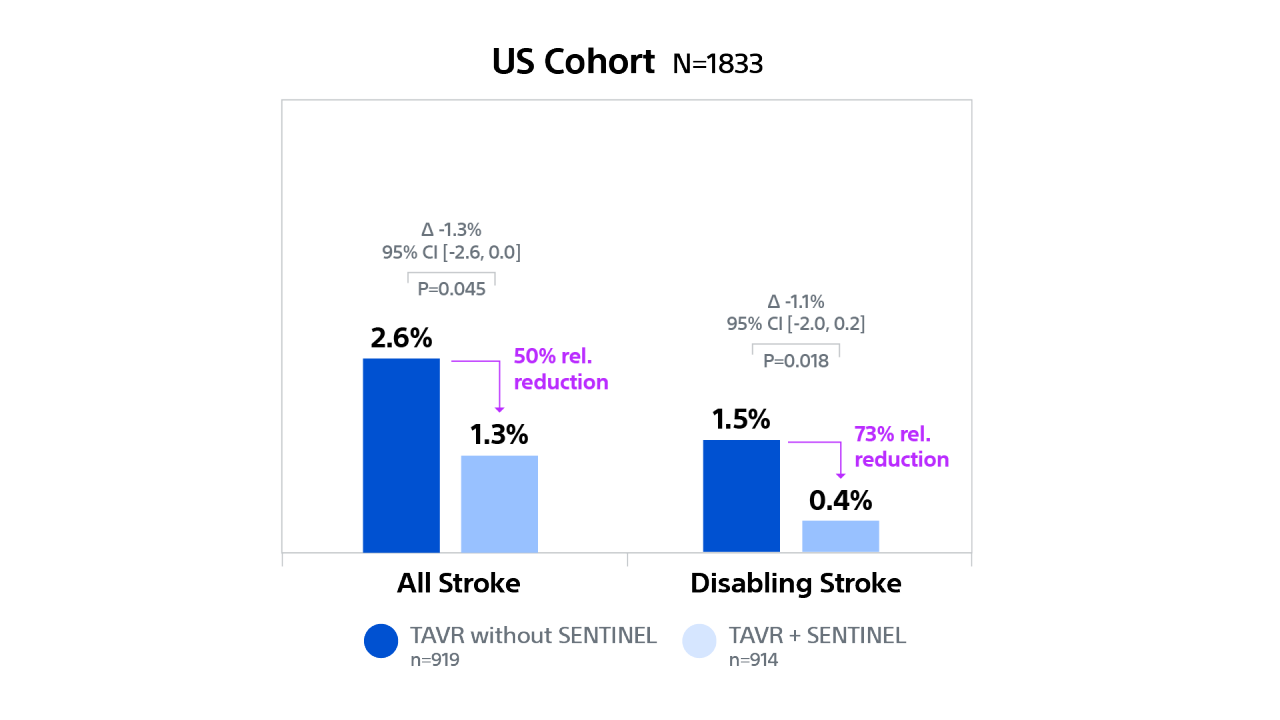
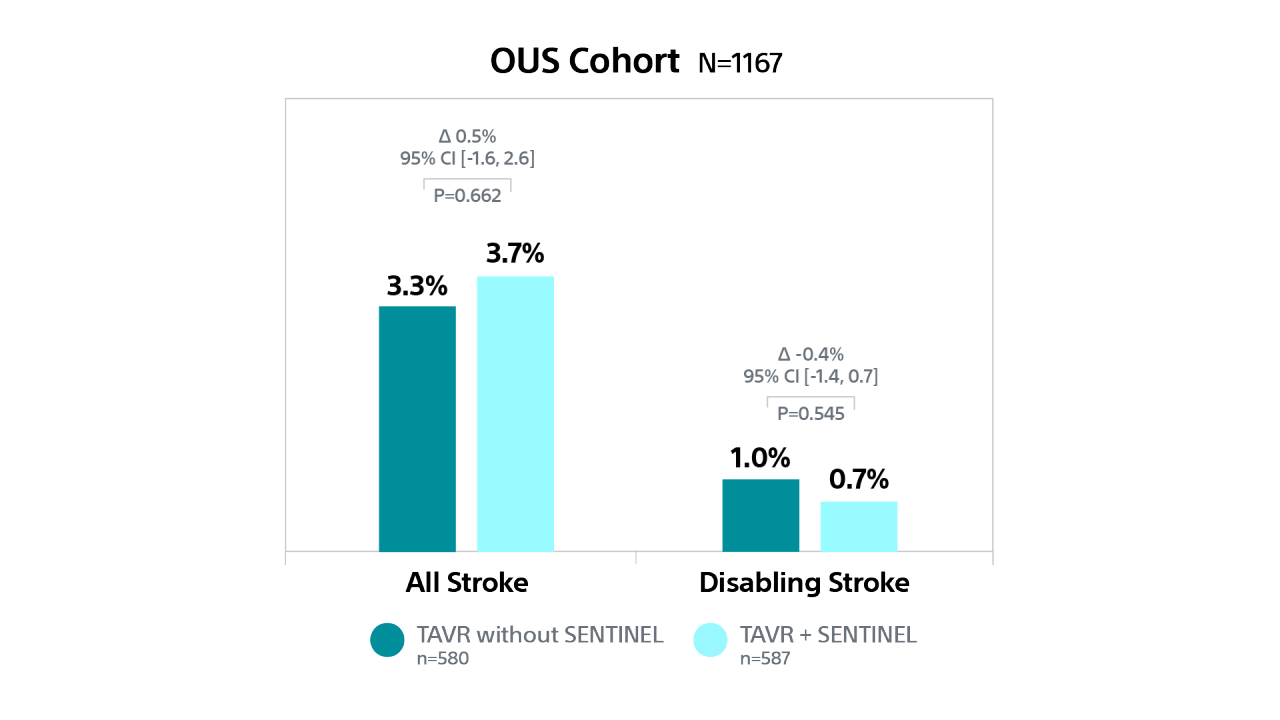
PTAVR Post-hoc Analysis: Outcomes by Geographic Region
Cerebral Embolic Protection by Geographic Region21,22
PROTECTED TAVR (PTAVR) is a randomized control trial (RCT) including N=3,000 patients with aortic stenosis enrolled at 51 sites globally and randomized 1:1 to TAVR with SENTINEL (n=1,501) or TAVR without SENTINEL (control group, n=1,499).
In US patients, TAVR with SENTINEL was associated with a 50% relative risk reduction for overall stroke and a 73% relative risk reduction for disabling stroke compared to TAVR without SENTINEL at discharge or 72 hrs.
Stroke Outcomes by Geographic Region
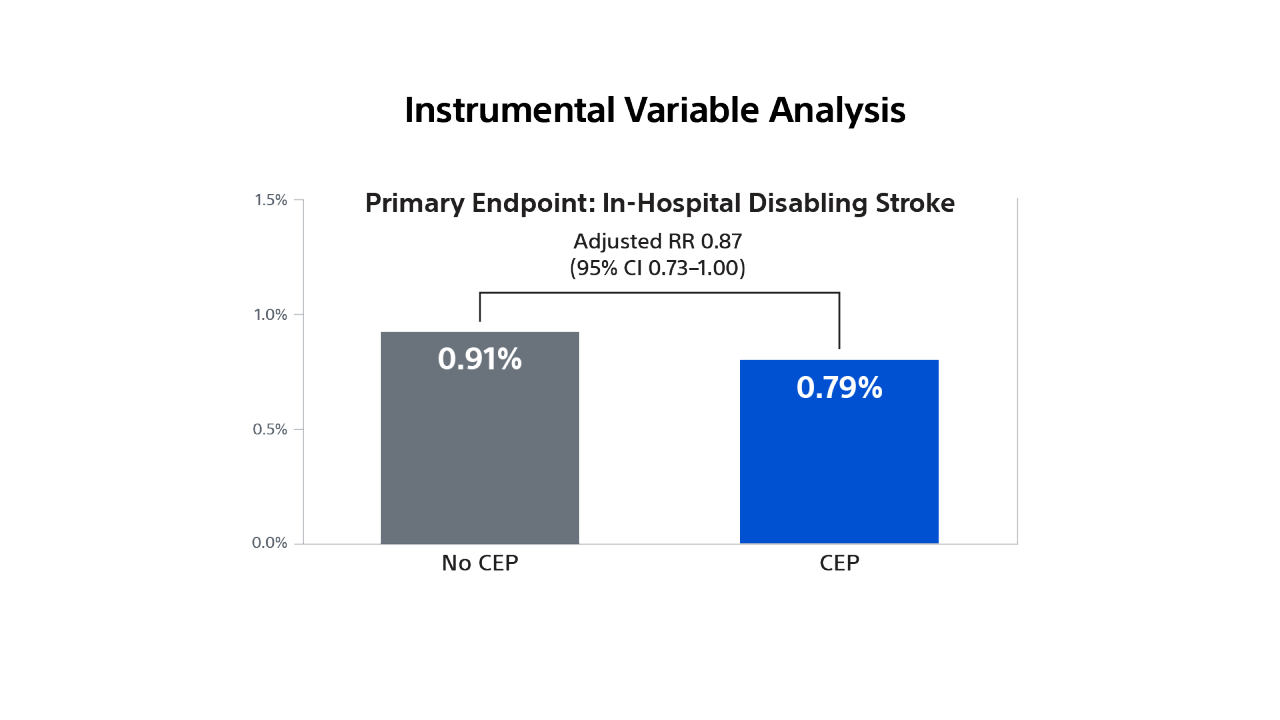
STS/ACC TVT Registry
Impact of Cerebral Embolic Protection Devices on Disabling Stroke after TAVR: Results from the TVT Registry23
Use real-world data to investigate whether use of SENTINEL is associated with reduction in disabling stroke in patients undergoing TAVR in contemporary practice.
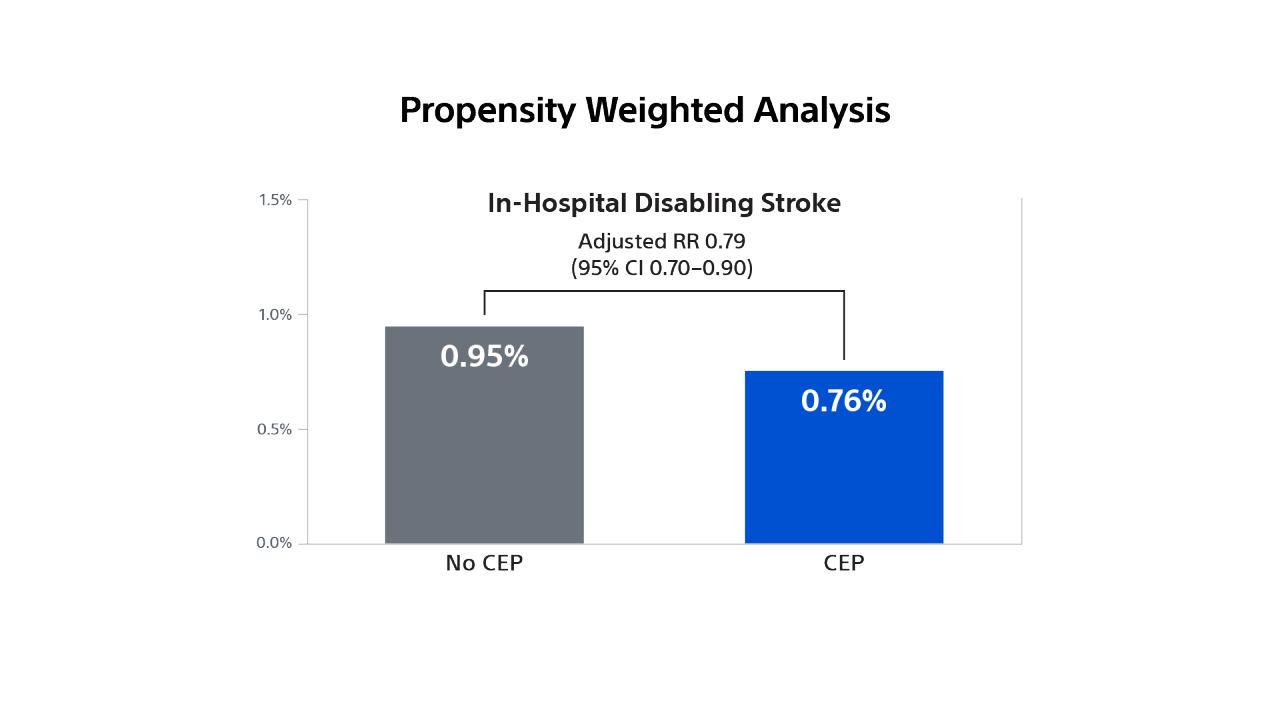
The PROTECTED TAVR Study
The PROTECTED TAVR Study
To demonstrate that use of the SENTINEL CPS significantly reduces the risk of peri-procedural stroke (≤ 72-hours) after TAVR.
Demonstrated a numerical trend towards a lower rate of stroke in patients treated with the SENTINEL device, representing a 21% relative risk reduction in all stroke through 72-hours or time of hospital discharge.
Statistically significant 60% relative risk reduction in disabling stroke through 72-hours or time of hospital discharge in patients treated with the SENTINEL device.
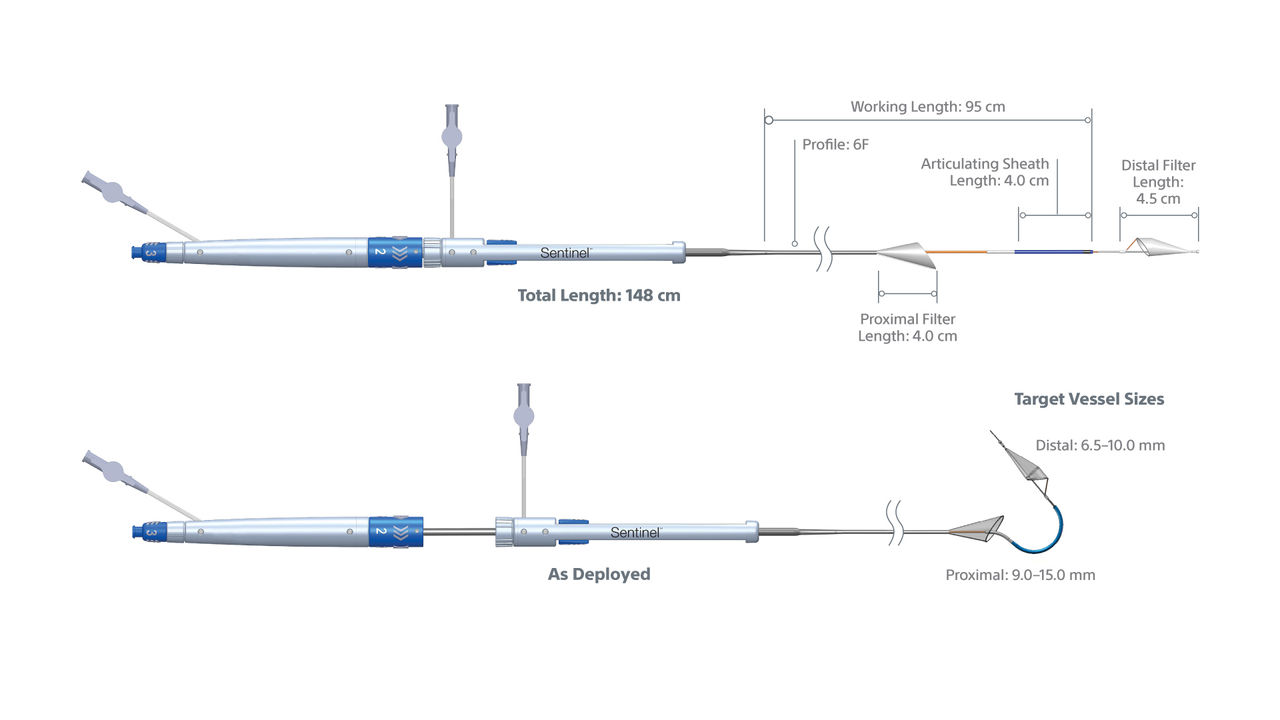
SENTINEL CPS Specifications
| Indications for use | The SENTINEL System is indicated for use as an embolic protection device to capture and remove embolic material (thrombus/debris) that may enter the cerebral vascular system during endovascular procedures.* |
|---|---|
Access Route | Right Radial Access Only (6F Radial Access Introducer Sheath) |
Deployment Procedure | Sequential Filter Deployment and Retrieval |
Guidewire Compatibility | 0.35 mm (0.014") Diameter Floppy Tip Coronary Guidewire, 175 cm Minimum Length |
| Use | Single Use |
Sterilization | E-beam Radiation |
Carton Size | 61.55 in x 1.05 in x 3.55 in |
Shelf Life | 2 Years |
Storage Information | Store at room temperature |
CPT code for cerebral embolic protection
Effective January 1, 2022, the use of SENTINEL cerebral embolic protection during TAVR procedures is an add-on treatment that qualifies for physician reimbursement.
| Indications for use | Order Number (GTIN) | Proximal Filter Size (mm) | Target Proximal Vessel Size (mm) | Distal Filter Size (mm) | Target Distal Vessel Size (mm) | Quantity |
|---|---|---|---|---|---|---|
CMS15-10C-US | 00863229000004 | 15.0 | 9.0-15.0 | 10.0 | 6.5-10.0 | 1 |
Resources
What do TAVR Patients Fear Most
Rose Hansen, VCC, and Dr. David Rose, Neurologist, discuss why patients fear stroke more than any other TAVR consequence.
Why I Believe in SENTINEL™
Dr. Ramon Quesada (IC), Dr. David Rose (neurologist), and Rose Hansen (VCC) tell us why they believe in Protected TAVR™ with SENTINEL.
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
1. Kapadia SR, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;69:367-377.
2. Kawakami R, et al. Characterization of cerebral embolic capture using the SENTINEL device during transcatheter aortic valve implantation in low to intermediate-risk patients: The SENTINEL-LIR Study. Circ Cardiovasc Interv. 2022;15: e011358. CIRCINTERVENTIONS.121.011358.
3. Schmidt T, et al. Debris heterogeneity across different valve types captured by a cerebral protection system during transcatheter aortic valve replacement. JACC Cardiovasc Intv. 2018;11:1262–1273.
4. Seeger J, et al. Significant differences in debris captured by the SENTINEL dual-filter cerebral embolic protection during transcatheter aortic valve replacement among different valve types. JACC Cardiovascular Interv. 2018;11:1683-1693.
5. Boston Scientific data on file.
6. Manoharan G, et al., J Am Coll Cardiol Intv 2015; 8:1359-67.
7. Wendler O, et al., Circulation 2017; 135: 1123–1132.
8. Seeger J, et al., Eur Heart J. 2018 Dec 24. doi: 10.1093/eurheartj/ehy847.
9. Haussig S et al., JAMA 2016; 316:592–601.
10. Kapadia S, Kodali S, Makkar R, et al., Protection against cerebral embolism during transcatheter aortic valve replacement. JACC. 2017; 69(4): 367–377.
11. Kapadia, et al. New England Journal of Medicine, 387(14), 1253–1263. Sep 2022.
12. Tsao CW, et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association external icon. Circulation. 2022;145(8):e153–e639.
13. Audience survey, Hawkey M, presented at ACC 2016.
14. Coylewright M, Palmer R, O’Neil E, et al., Patient-defined goals for the treatment of severe aortic stenosis: A qualitative analysis. Health Expect. Oct 2016:19(5): 1-36-1043.
15. Alkhouli, M, et al. “Cost of procedural stroke in TAVR in a US Medicare population” Valve20A-POS01; PCR London Valves 2020.
16. Kapadia, S. PROTECTED TAVR Trial data presented at TCT 2022.
17. SENTINEL IDE trial. Data presented at SENTINEL advisory panel, February 23rd, 2017.
18. SENTINEL IDE Trial. Data presented at SENTINEL FDA Advisory Panel, Feb 23, 2017.; 2. Van Mieghem N., TVT 2018 (includes TIA); 3. Stripe, B. PCR LV 2019; 4. Rinaldi, TCT 2018. 5. Chakravarty T., TCT 2018; 6. Seeger J., et al., JACC Cardiovasc Interv. 2017.
19. All photographs taken by Boston Scientific. Illustrations for informational purposes only – not indicative of actual size or clinical outcome.
20. Kharbanda RK. Et al. Routine Cerebral Embolic Protection during Transcatheter Aortic- Valve Implantation. NEJM. 2025.
21. Makkar RR. et al. Cerebral Embolic Protection by Geographic Region. A Post Hoc Analysis of the PROTECTED TAVR Randomized Clinical Trial. JAMA Cardiology. 2024.
22. Kapadia SR. et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. NEJM. 2022.
23. Neel Butala et al. Cerebral Embolic Protection and Outcomes of Transcatheter Aortic Valve Replacement: Results from the TVT Registry. Circulation. 2021.
24. Mullen MJ, Ludman PF, Banning AP, et al. Routine cerebral embolic protection during transcatheter aortic-valve implantation. N Engl J Med. 2025;392(13):1234–1244. doi:10.1056/NEJMoa2403264.