IMPERIAL randomized controlled trial results
Eluvia DES demonstrated statistically significant difference in primary patency versus Zilver PTX and achieved the highest primary patency reported in any SFA DE Pivotal Trial at 1-Year.7,8,9,10
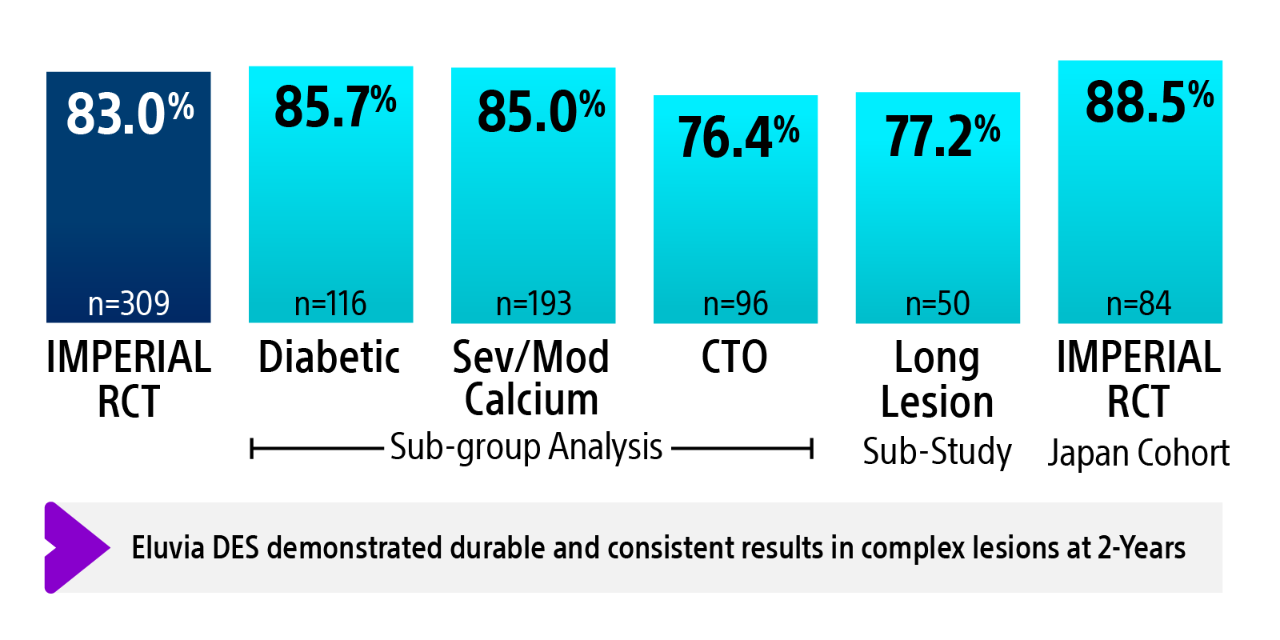
The IMPERIAL RCT demonstrated that Eluvia DES is clinically effective and safe in treating patients with symptomatic SFA disease both in the short-term during the height of restenosis risk, and long-term out to five years.
IMPERIAL RCT 1-year primary patency results

IMPERIAL RCT 2-year primary patency results¹ʼ²
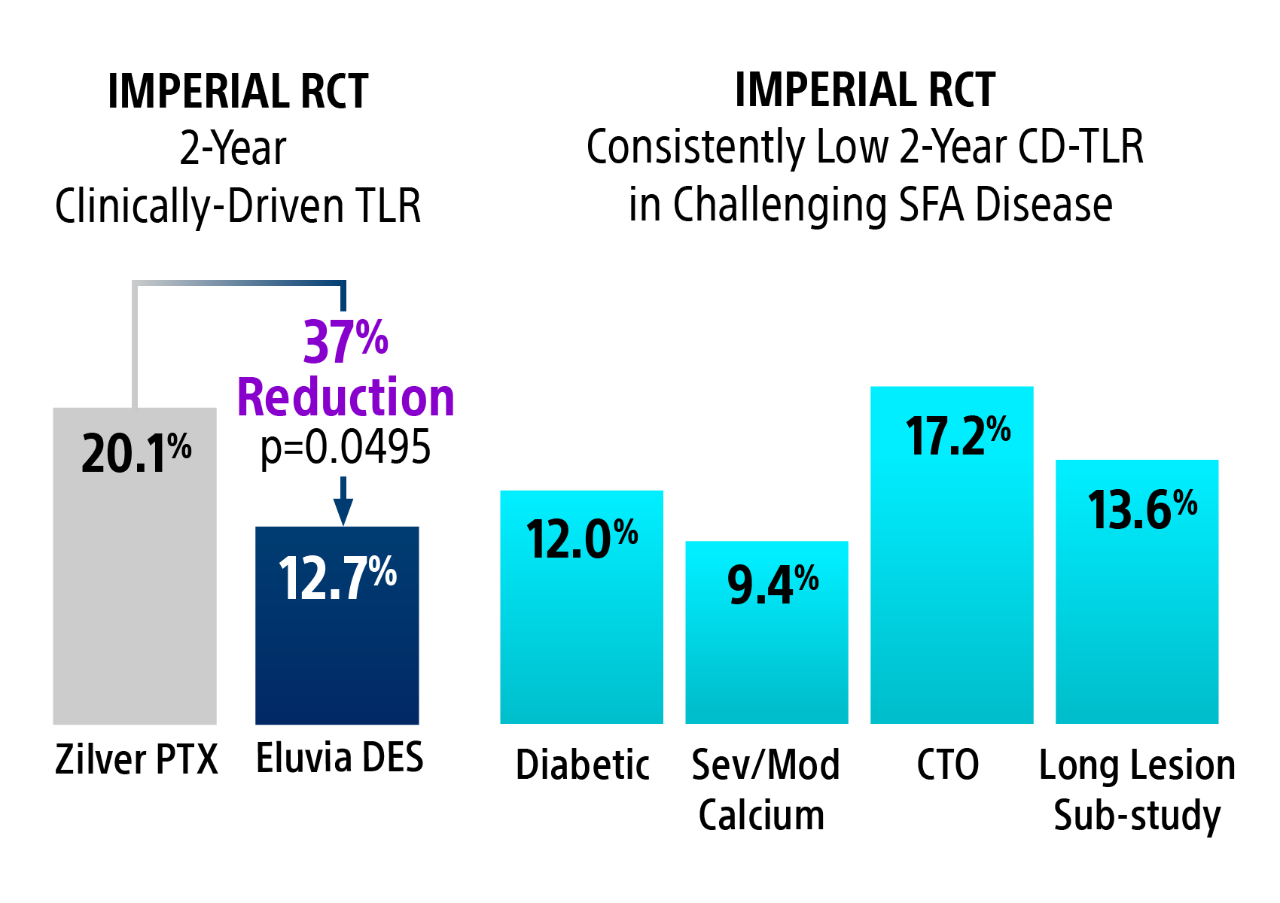
2-year clinically-driven TLR
Eluvia DES had statistically significant fewer CD-TLRs compared to Zilver PTX at 2-Years.16
IMPERIAL randomized controlled trial details
- 2-year primary endpoints
- Mortality rates
- Baseline characteristics
| 2-Year Primary Endpoints | Eluvia DES (n=309) | Zilver PTX (n=156) | p-value |
| Primary Patency | 83.0% | 77.1% | 0.1008 |
| Major Adverse Events | 14.2% | 20.1% | 0.1236 |
| Mortality Rates | Eluvia DES | Zilver PTX | p-value |
|---|---|---|---|
| 1-Year All-Cause Mortality | 2.1% | 4.0% | 0.23 |
| 2-Year All-Cause Mortality | 7.1% (21/295) | 8.3% (12/145) | 0.6649 |
| Baseline Characteristics | Eluvia DES | Zilver PTX |
| Age (Years) | 68.5 ± 9.5 | 67.8 ± 9.4 |
| Male Gender | 66.0% | 66.7% |
| Diabetes Mellitus | 41.7% | 43.6% |
| History of Smoking | 86.1% | 84.0% |
| Target Lesion Length (mm) | 86.5 ± 36.9 | 81.8 ± 37.3 |
| Severely Calcified | 40.1% | 32.3% |
| Total Occlusions | 31.2% | 30.3% |
| Extending into Distal SFA | 66.3% | 65.4% |
