Boston Scientific accounts are for healthcare professionals only.




WaveWriter Alpha™ Spinal Cord Stimulator System
Reimbursement
Configure or select a product to continue to order
- Overview
- Therapy
- Clinical data
- Technical specifications
- Ordering information
- Training
- Resources
PROFOUND.
PROVEN.
DIFFERENT.
SCS Therapy That Succeeds Where Others Fail.
Boston Scientific offers the broadest SCS portfolio, and an exclusive sub-perception therapy that delivers pain relief in 2 minutes and lasts for years.1-5†
†Disclaimer: These outcomes reflect approximated data as determined in the referenced studies.
How it works
The WaveWriter Alpha SCS System is the most comprehensive SCS portfolio to allow for full-body MRI* access.‡
Why the WaveWriter Alpha SCS System is right for your practice
The WaveWriter Alpha™ Spinal Cord Stimulator (SCS) System is a comprehensive portfolio of devices that gives you and your patients more control over managing chronic pain symptoms.
Key benefits:
- Featuring FAST™ Therapy (Fast-Acting Sub-Perception Therapy), the only SCS therapy that delivers profound, paresthesia-free pain relief in minutes and continues to provide relief for years.1-5
- Offering a proven solution backed by clinical evidence from 4 Level I randomized controlled trials (RCTs) and 4 real-world studies.
- Providing technology that is different by design, the WaveWriter Alpha™ SCS System includes a full-body MRI-conditional* portfolio of rechargeable and primary-cell implantable pulse generators (IPGs) (16 and 32 contacts) with multi-lumen percutaneous leads and paddles.
- Delivering all setting options through a discrete, easy-to-use mySCS™ GO Therapy Controller for safe, simple adjustment.
Subscribe for updates on the WaveWriter Alpha SCS System
Receive timely access to major announcements, opportunities to connect with your peers through educational events, and useful tools for you to help more patients.
FAST™ Therapy delivers profound, paresthesia-free pain relief in minutes that lasts for years1-5
With conventional sub-perception SCS therapy, it takes time to determine whether your patient will experience pain relief and a slow 2-day wash-in can waste ~30% of a 7-day trial.*
*2 days is approximately 30% of a 7-day trial per 2 days / 7 days = 0.285 ≈ 30%.
FAST Therapy allows quick confirmation that your patients are experiencing relief.
Profound pain relief
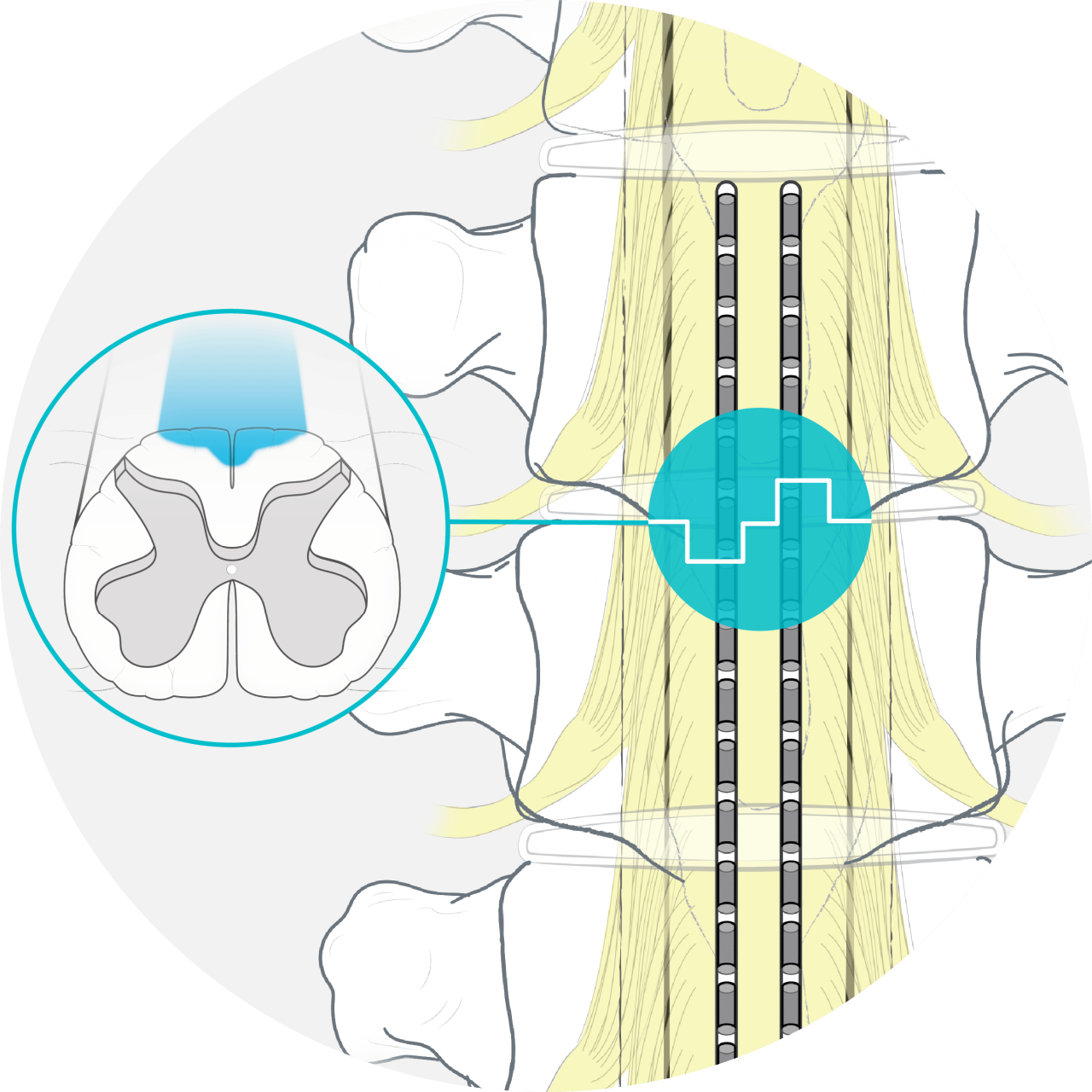
How FAST Therapy works
FAST engages Surround Inhibition, a novel mechanism of action in SCS, using Boston Scientific’s proprietary technologies for precise targeting and dosing.
Precise target +
Proper neural dose =
FAST Therapy
Our latest real-world study demonstrates that patients who preferred FAST Therapy achieved significant pain relief sustained out to 3 years.6,7
The clinical data behind FAST Therapy
Minutes to Pain Relief2,3†
Pain Score3,4,5†
Year Outcomes6,7
Clinical data shows that FAST is
Profound
5.6-point pain score reduction on NRS scale out to 2 years5
29-point improvement in disability on ODI scale out to 2 years5
Preferred
Physicians and patients prefer to achieve pain relief in minutes instead of days3
Boston Scientific's SCS therapy
Boston Scientific’s multiple mechanism SCS therapy is backed by Level I RCTs and Real-world Studies
Featured FAST™ SCS Therapy clinical data: 2–3-year outcomes
5.6-point reduction in pain score out to 2 years9
5.0-point reduction in pain score out to 2 years4
6.1-point reduction in pain score out to 3.6 years6
Technical specifications
| FEATURE | WAVEWRITER ALPHA SCS SYSTEM | WAVEWRITER ALPHA 16 | WAVEWRITER ALPHA PRIME | WAVEWRITER ALPHA PRIME 16 |
|---|---|---|---|---|
| Power Sources | 32 | 16 | 32 | 16 |
| Volume | 21.6cc | 20.1cc | 36.6cc | 34.9cc |
| Thickness | 10.7mm | 10.7mm | 11.6mm | 11.6mm |
| Battery Type | Rechargeable | Rechargeable | Non-Rechargeable | Non-Rechargeable |
| Battery Capacity | 200mAh | 200mAh | 7Ah | 7Ah |
| Wireless Recharging | Yes | Yes | N/A | N/A |
| Wireless Remote | Yes | |||
| Remote Range | 3m | |||
| Bluetooth Enabled | Yes | |||
| MRI Conditionality | Full Body* | |||
| System Type | Multiple Independent Current Control (MICC) at 1% increments | |||
| FDA Labeling | FDA approved for both paresthesia and paresthesia-free therapies | |||
| Advanced Paresthesia and Paresthesia-Free Therapies | FAST Therapy, Contour Therapy, Illumina 3D™ Standard Rate, High Rate 3D, MicroBurst 3D, Burst 3D, Prism™ Targeting | |||
| Simultaneous Combination Therapy | Yes | |||
| Amplitude | 0-25.5mA | |||
| Rate | 2-1200Hz | |||
| Pulse Width | 20-1000μs | |||
| Upgradeable | Yes | |||
Ordering information
| MODEL NUMBER | UPN | DESCRIPTION |
|---|---|---|
| SC-1416 | M365SC14160 | WaveWriter Alpha Prime 16 Implantable Pulse Generator Kit |
| SC-1432 | M365SC14320 | WaveWriter Alpha Prime Implantable Pulse Generator Kit |
| SC-1216 | M365SC12160 | WaveWriter Alpha 16 Implantable Pulse Generator Kit |
| SC-1232 | M365SC12320 | WaveWriter Alpha Implantable Pulse Generator Kit |
| SC-5275 | 9258388902 | mySCS GO Therapy Controller |
| SC-6500-72 | M365SC6500720 | WaveWriter Alpha Patient Trial Kit |
Training for WaveWriter Alpha SCS System
Duration: 40:30
Date: January 2021
Duration: 1:07:15
Date: January 2021
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Resources on ImageReady™ MRI
The WaveWriter Alpha Spinal Cord Stimulator (SCS) System is the most comprehensive portfolio in SCS with full-body MRI* access‡.

Boston Scientific offers the first-and-only MRI conditional adapters allowing connection to leads from other manufacturers
Connect to Medtronic and Abbott trials and implants**
M8 and S8 MRI Conditional Adapters
Direct connect for Nevro trials and implants§
MRI conditionality not evaluated for direct connect
Information on expanded indications for SCS
Approved for the treatment of chronic pain associated with DPN
Real-world DPN evidence at 2 years10
A sub-analysis of Boston Scientific's real-world RELIEF registry demonstrated a high responder rate of 81% and 95% sustained improvement at 2 years.11
81% responder rates 95% sustained patient improvement
Approved for the treatment of chronic pain associated with DPN
The SOLIS randomized control trial demonstrates that the WaveWriter Alpha™ SCS System delivered superior outcomes compared to conventional medical management (CMM) alone and sustained outcomes out to one year.12
~90% sustained responder rate over 1-year 86% patient satisfaction in the CMM arm at 3-months
Unlock more targeted treatment options for your chronic pain patients
To explore our comprehensive portfolio of therapies for chronic pain, visit BostonScientific.com/Pain.
References:
For a list of compatible mobile devices, visit www.myscsjourney.com/mySCSGO. The mySCS™ GO Therapy Controller Application is compatible with the WaveWriter Alpha Spinal Cord Stimulation System only.
*MRI Conditional under specified conditions
** Refer to the Precision M8 Adapter Directions for Use for indications, warnings and cautions. The Precision M8 Adapter is compatible with the following Medtronic lead and extension model numbers: 39286, 39565, 3778, 3878, 3777, 3877, 3776, 3876, 3874, 3873, 3875, 37081, 37083, 37082, 977A160, 977A175, 977A190, 977A260, 977A275, 977A290, 977D160, 977D260 Refer to the “Precision™ S8 Adapter Directions for Use” for indications, warnings and cautions. The Precision™ S8 Adapter is compatible with the following St Jude Lead, Adapter and Extension model numbers: 3283, 3268, 3269, 3280, 3244, 3262, 3263, 3245, 3264, 3265, 3208, 3210, 3288, 3289, 3214, 3219, 3228, 3183, 3186, 3189, 3191, 3086, 3286, 2283, 3341, 3342, 3343, 3346, 3382, 3383, 3386.
†Disclaimer: These outcomes reflect approximated data as determined in the referenced studies.
‡ “ImageReady™ MRI Full Body Guidelines for WaveWriter Alpha™ and WaveWriter Alpha™ Prime Spinal Cord Stimulator Systems” & “ImageReady™ MRI Full Body Guidelines for WaveWriter Alpha™ and WaveWriter Alpha™ Prime Spinal Cord Stimulator Systems with Adapters”. Refer to https://www.bostonscientific.com/elabeling/us/en/home.html for more information. MRI Guidelines for Medtronic Neurostimulation Systems for Chronic Pain. M939858A010 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the SENZA®, SENZA II® & SENZA Omnia™ Systems. 11096 MRI Procedure Information For Abbott Medical MR Conditional Neurostimulation Systems. ARTEN600090483 A
§The Boston Scientific SCS Implantable Pulse Generators, OR Cables, and Lead Extensions can be connected to the following compatible Nevro Leads and Lead Extensions: LEAD2008-25B, LEAD2008-35B, LEAD2008-60B, LEAD1058-30B, LEAD1058-50B, LEAD1058-70B, LEAD1058-90B, TLEAD1058-50B, TLEAD1058-70B, TLEAD1058-90B. Warning: The Nevro Leads and Lead Extensions have not been tested for compatibility with an MRI environment when used with the Boston Scientific SCS Systems.
Warning: Stimulation modes. Only paresthesia-based stimulation mode has been evaluated for effectiveness in the diabetic peripheral neuropathy (DPN) population
Sub-perception stimulation has been demonstrated to be safe and effective in patients who have been treated successfully with conventional, paresthesia-inducing stimulation for at least six months. Full stimulation parameter ranges and options for both paresthesia based and sub-perception therapy are available for clinician’s use throughout the patient’s experience and treatment with SCS.
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
- Tsang et al. Reducing surgical interventions using spinal cord stimulation leads providing long span and tight contact spacing. [Abstract] NANS 2025 Annual Meeting, Jan 30-Feb 1, 2025. *compared to a wide-spaced 8-contact lead.
- Gage E., et al. Rapid Onset of Analgesia during Trial Period Utilizing Fast-Acting Sub-Perception Therapy SCS [Abstract] NANS 2023 Annual Meeting, Jan 12-15, 2023. (n=44)
- Anitescu M., et al. Utilizing SCS Designed to Engage Surround Inhibition Using Fast-Acting Sub-Perception Therapy (FAST): Prospective, Multicenter, Long-Term Outcomes [Abstract] NANS 2025 Annual Meeting, Jan 30-Feb 1, 2025.
- Metzger C, Hammond B, Ferro R, et al. Two-year outcomes using fast-acting sub-perception therapy for spinal cord stimulation: results of a real-world multicenter study in the United States. Expert Rev Med Devices. 2025;22(2):155-164. doi:10.1080/17434440.2025.2453554. (n=50)
- Bayerl S., et al. Clinical Outcomes Using A New Fast-Acting Sub-Perception Therapy For Chronic Pain: A Multicenter European Observational Real-World Study [Abstract]. Twenty-Sixth Annual Meeting of the North American Neuromodulation Society, January 12-15, 2023.
- Ferro R, North J, Kranenburg A, et al. Automated neural dosing for fast-acting subperception therapy: sustained spinal cord stimulation outcomes from a multicenter study. Pain Ther. 2025;14(4):1417-1429.
- Bayerl S., et al. Long-term Outcomes of Fast-acting Sub-perception SCS: Multinational, Real-world Analysis across Europe and the United States. [Abstract] NANS 2026 Annual Meeting, Jan 22-25, 2026.
- Ferro R. et al. Streamlining Spinal Cord Stimulation Therapy via Personalized Automation of Programming [Abstract] NANS 2025 Annual Meeting, Jan 30-Feb 1, 2025. (n=23)
- Bayerl S, Paz-Solis J, Matis G, et al. Two-year outcomes using fast-acting, sub-perception therapy for spinal cord stimulation: a European, real-world, multicenter experience. J Clin Med. 2024;13(22):6999. doi:10.3390/jcm13226999. (n=52)
- Berg A et al. Global, Multicenter Registry of Prospectively-Enrolled Patients Utilizing SCS for Painful-Diabetic Peripheral Neuropathy (DPN): Long-Term Outcomes [Abstract]. Twenty-seventh Annual Meeting of the North American Neuromodulation Society, January 18-21, 2024.
- Rauck R. et al. Real-World Outcomes Using Spinal Cord Stimulation for Treatment of Chronic Back Pain with No Prior Back Surgery [Abstract 88]. Third joint Congress of the INS European Chapters, August 31-September 2nd, 2023, (n=48)
- North J., et al. Evaluating SCS and Medical Management for Chronic Pain Without Prior Surgery: SOLIS RCT 24-Month Outcomes [Abstract], NANS 2025 Annual Meeting, Jan 30- Feb l, 2025. (n=77)


