Overview
The ADVENT trial is the first randomized clinical trial that directly compared FARAPULSE™ PFA (with the FARAWAVE™ Pulsed Field Ablation (PFA) Catheter) to standard-of-care thermal ablation—radiofrequency ablation (RFA) and cryoballoon ablation (CBA)—for the treatment of paroxysmal atrial fibrillation (PAF).
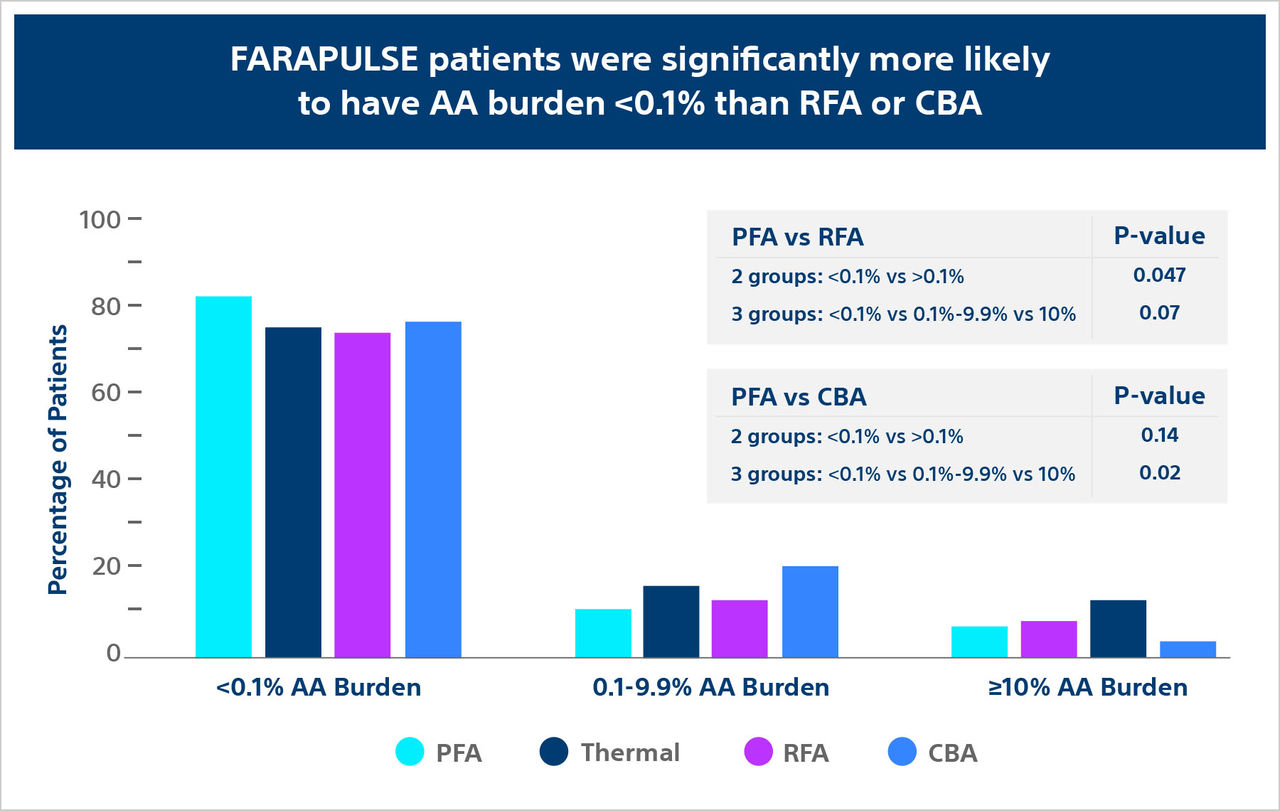
The ADVENT trial: AA burden sub-analysis compared FARAPULSE PFA (with the FARAWAVE PFA Catheter) to standard-of-care thermal for recurrent atrial arrythmia (AA).
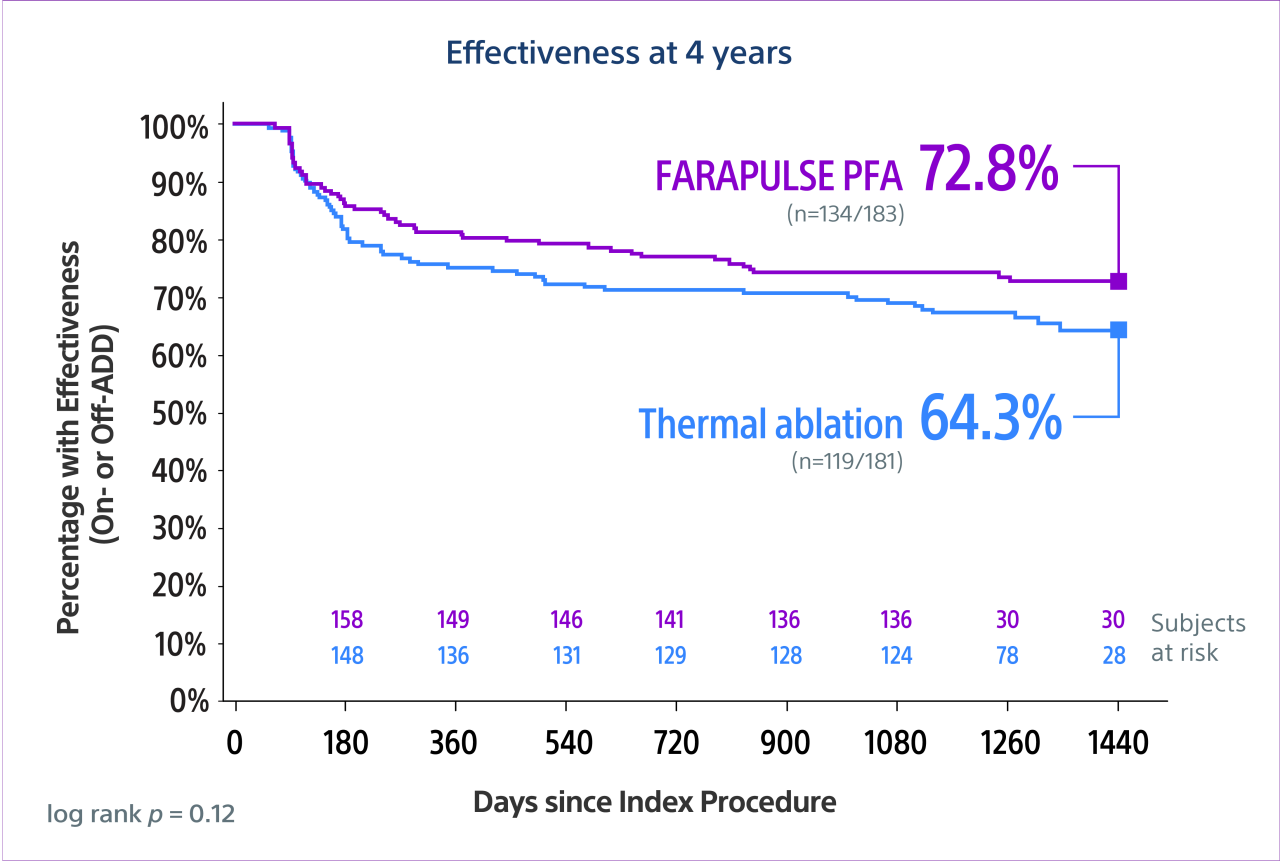
The ADVENT long-term outcomes (LTO) study—an observational extension of the pivotal ADVENT trial—assessed effectiveness of FARAPULSE PFA (with the FARAWAVE PFA Catheter) vs thermal (RF or cryo) ablation out to 4 years.