AMS 700™ Inflatable Penile Implant
Important Safety Information
Tactra™ Malleable Penile Implant
Important Safety Information
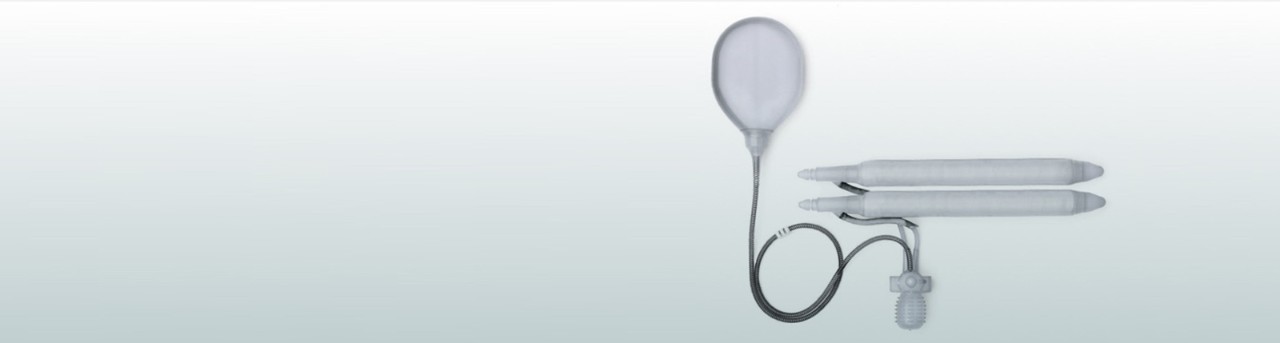
AMS Ambicor™ Penile Implant
Your doctor is your best source for information on the risks and benefits of the AMS Ambicor™ Inflatable Penile Prosthesis. Talk to your doctor for a complete listing of risks, warnings and important safety information.
The AMS Ambicor™ Inflatable Penile Prosthesis is intended for use in the treatment of male erectile dysfunction (impotence). Implanting a penile prosthesis will damage or destroy any remaining ability to have a natural erection as well as make other treatment options (oral medications, vacuum devices or injections) impossible.
Men with diabetes, spinal cord injuries or skin infections may have an increased risk of infection. Implantation may result in penile curvature or scarring.
Potential risks may include: device malfunction/failure leading to additional surgery, device migration potentially leading to exposure through the tissue, wearing away/loss of tissue (device/tissue erosion) infection, unintended-inflation of the device and pain/soreness.
Spectra™ Concealable Penile Implant
Your doctor is your best source for information on the risks and benefits of the Spectra™ Concealable Penile Prosthesis. Talk to your doctor for a complete listing of risks, warnings and important safety information.
The Spectra™ Concealable Penile Prosthesis is intended for use in the treatment of male erectile dysfunction (impotence). Implanting a penile prosthesis will damage or destroy any remaining natural ability to have a natural erection, as well as make other treatment options impossible.
Men with diabetes, spinal cord injuries or skin infections may have an increased risk of infection. Implantation may result in penile shortening, curvature or scarring.
Additional information is provided in the product Patient Manuals, available through your doctor.