Boston Scientific accounts are for healthcare professionals only.
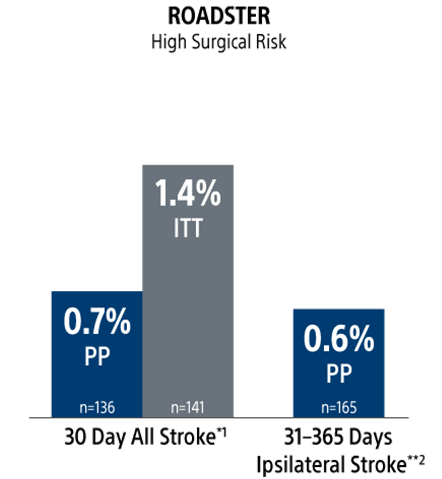
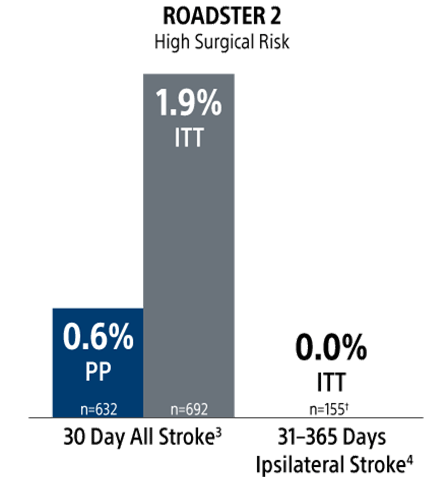
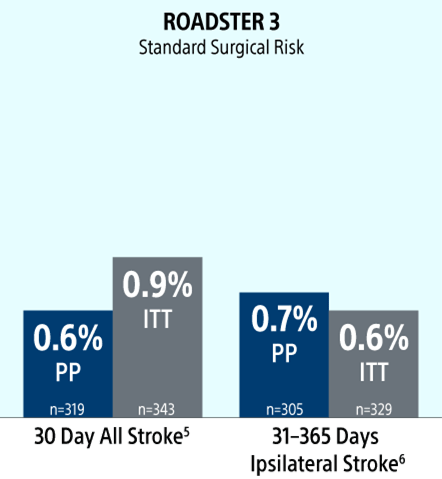
The ROADSTER trials: Proven and Predictable Outcomes Out To 1-Year Regardless of Patient Risk Status
ROADSTER 3 is the first independently adjudicated, prospective study evaluating TCAR in an exclusive standard surgical risk population. Results demonstrate that TCAR is safe and effective in standard surgical risk patients with excellent clinical outcomes at 30 days and out to 1-Year.1
Stroke Rates
ITT = intent to treat
PP = per protocol
References:
* Lead-in + pivotal phases.
** Pivotal + extended phases.
† Long-term follow up cohort
1. Kwolek CJ, et al. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J Vasc Surg. 2015 Nov;62(5):1227–34.
2. Malas MB, et al. Analysis of the ROADSTER pivotal and extended-access cohorts shows excellent 1-year durability of transcarotid stenting with dynamic flow reversal. J Vasc Surg.2019;69(6):1786–1796.
3. Kashyap VS, et al. Early Outcomes in the ROADSTER 2 Study of Transcarotid Artery Revascularization in Patients With Significant Carotid Artery Disease. Stroke. 2020 Sep;51(9):2620–2629.
4. Kashyap, VS. et al. One-year outcomes after transcarotid artery revascularization (TCAR) in the ROADSTER 2 trial. Journal of Vascular Surgery, Volume 76, Issue 2, 466–473.e1.
5. ROADSTER 3 30 Day Follow-up results presented by Meghan Dermody at VIVA 2024.
6. ROADSTER 3 1-Year Follow-up results presented by Meghan Dermody at VIVA 2025.