Methods
Between 2022–2024, 344 intent-to-treat (ITT) patients were enrolled (319 treated per-protocol (PP)) at 53 US sites (48 sites enrolled patients). The FDA analysis population was per protocol. Patients who underwent the study procedure but with major protocol deviations were excluded from the per protocol group. The primary endpoint for this single-arm, post-approval study was a composite of major adverse events (stroke, death, or myocardial infarction (MI)) through 30 days post-procedure, plus ipsilateral stroke from day 31 to 365 post-procedure. The incidence of cranial nerve injury (CNI) within 30 days post-procedure was a key secondary endpoint. Independent neurological assessments were performed for all patients before the procedure, within 24 hours, at 30-days, and at 1-year after TCAR. Events were adjudicated by an independent clinical events committee.
Findings
In the PP population, 75.5% of patients were less than 75 years of age, 42.3% were female, and 15.4% were symptomatic. Approximately 25.0% of symptomatic patients underwent a TCAR procedure within 2 weeks of their prior neurological event. The mean lesion length was 23.1mm, 47.7% had a Type II or Type III aortic arch, and 16.3% of lesions had severe calcification.
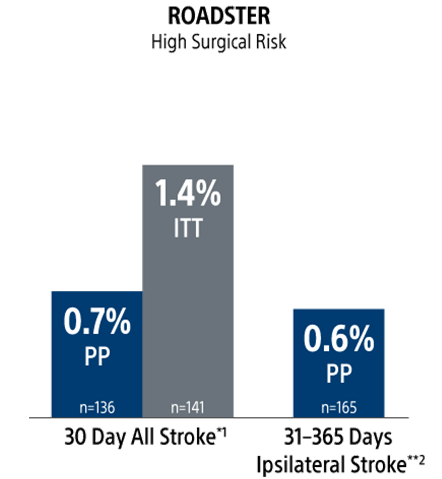
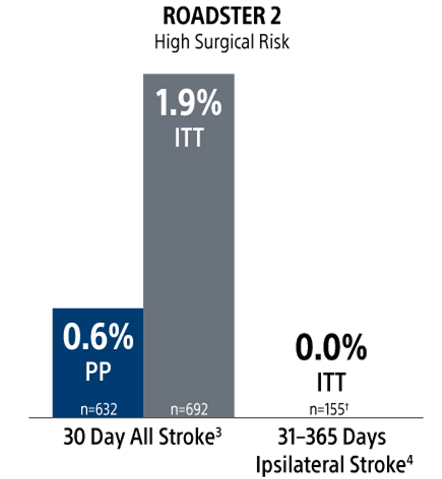
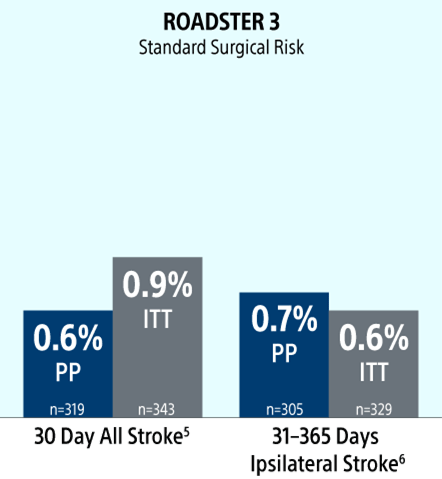
30-Day Results (Presented at VIVA 2024): In the PP population, the rate of stroke/death/MI at 30-days was 0.6% (0.9% ITT) with a 30-day stroke rate of 0.6% (2/319) [0.9% (3/343) ITT]. There were no deaths or MIs through 30-day follow-up. The incidence of CNI within 30 days was 0.6% (0.6% ITT); both resolved within 6 months.
1-Year Results (Presented at VIVA 2025): In the PP population, the rate of Major Adverse Events at 30-days (Stroke/Death/MI) plus ipsilateral stroke to 1-year was 1.3% (1.5% ITT). Ipsilateral stroke from 31 days to 365 days was 0.7% (2/305) [0.6% (2/329) ITT].
Conclusions
ROADSTER 3 is the first independently adjudicated, prospective study evaluating TCAR in an exclusive standard surgical risk population. Results demonstrate that TCAR is safe and effective in standard surgical risk patients with excellent clinical outcomes at 30 days and out to 1-Year.