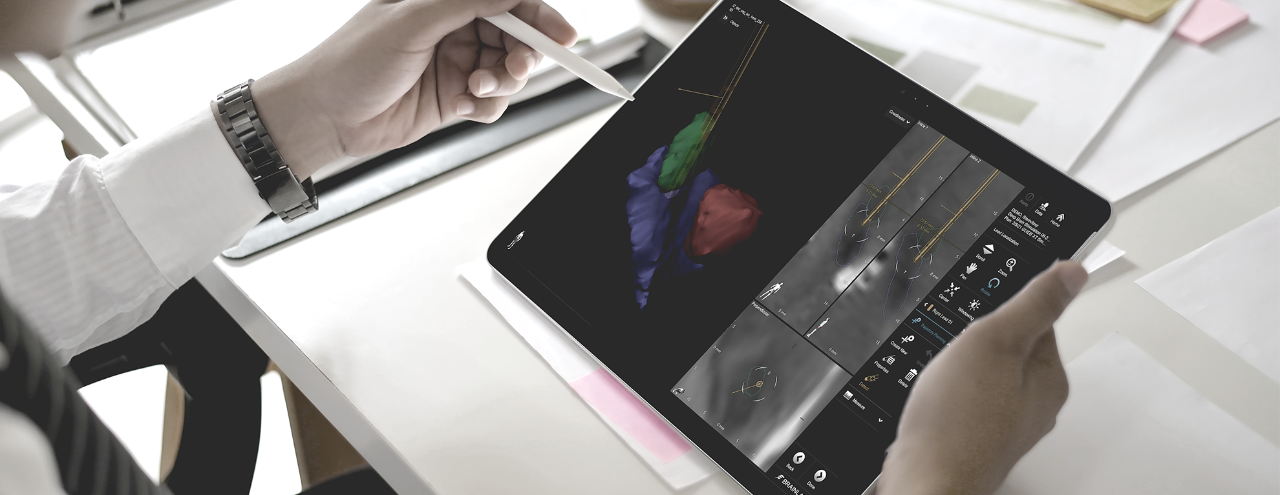
Use Brainlab Elements on the Boston Scientific DBS Clinician Programmer to assist during your programming session
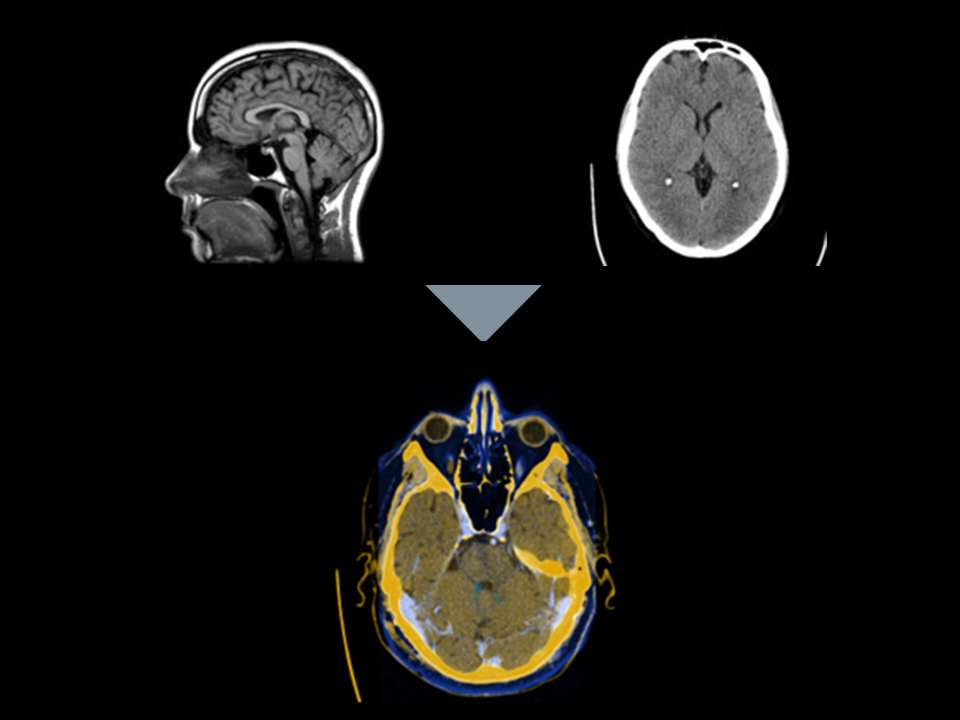
1. Image Fusion
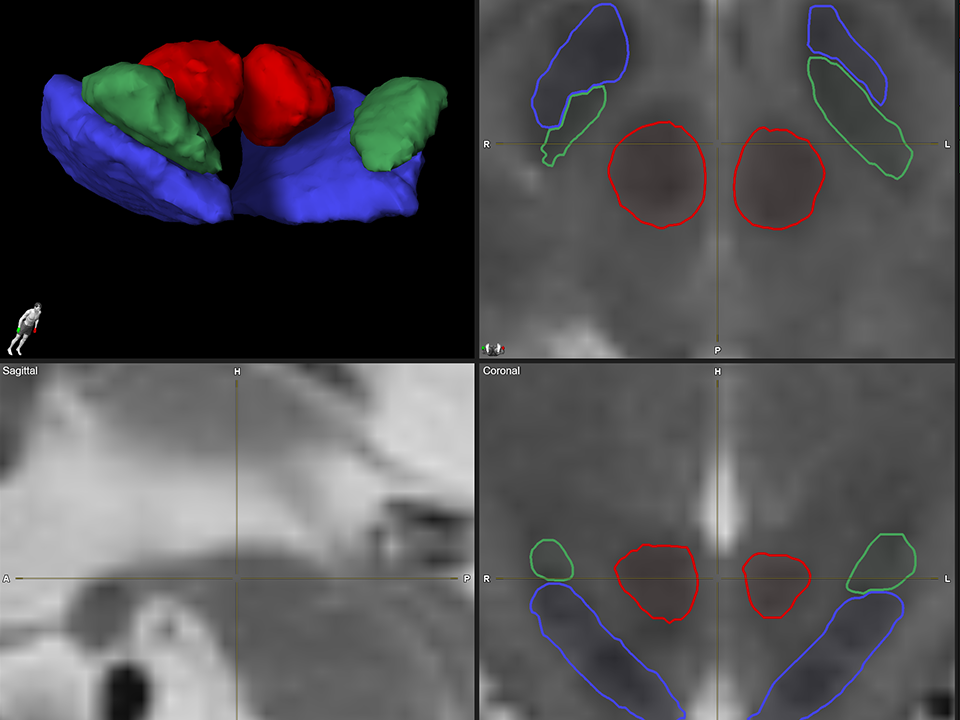
2. Anatomical Mapping
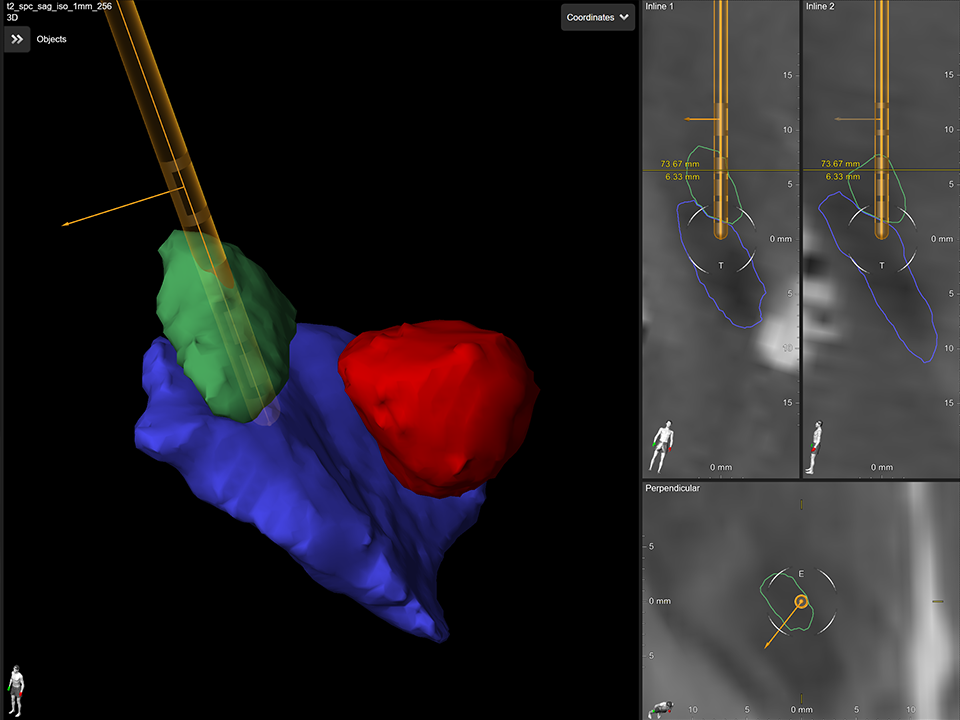
3. Lead Localization
1. Elements Image Fusion
Automatically fuse your patient’s MRIs and post-op CTs with Elements Image Fusion. Image Fusion automatically co-registers your patient imaging.
B. Easy to use tool to verify and accept fusion.
2. Elements Anatomical Mapping
Automatically visualize patient specific anatomy for the patient’s imaging with Elements Anatomical Mapping.
A. Highly consistent MRI-Based segmentation of structures in the basal ganglia region.
B. Proprietary adaptive algorithm captures inherent asymmetry and abnormality.
3. Elements Lead Localization
Automatically detect the location and orientation of the Vercise Cartesia™ Directional Lead based on post-op CT artifact and see, in 3D, the individual lead contacts in the patient’s own anatomy with Elements Lead Localization.
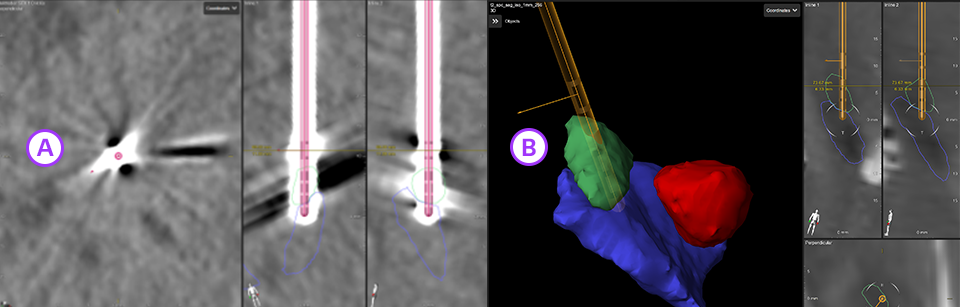
A. Image-based detection of lead location and orientation based on post-op CT artifact.
B. 3D display of the lead within the target anatomy.
Brainlab Elements
To learn more about the Brainlab Elements Image Fusion, Anatomical Mapping and Lead Localization, visit Brainlab's Online Campus.
Precision takes shape
Easily customize therapy to match each patient’s needs with the Vercise Genus™ DBS system.
Precision delivered
The Vercise Genus DBS System features directional and standard leads powered by Cartesia 3D.*
Clinical excellence
The Vercise™ DBS systems are supported by dozens of studies with thousands of patients worldwide showing clinical benefits.
Images provided by Brainlab
The Bluetooth® word mark and logos are registered trademarks owned by the Bluetooth SIG, Inc. and any use of such marks by Boston Scientific Neuromodulation Corporation is under license.
Brainlab Elements Image Fusion is an application for the co-registration of image data within medical procedures by using rigid and deformable registration methods. It is intended to align anatomical structures between data sets. The device itself does not have clinical indications.
The Brainlab Elements Trajectory Planning software is intended for pre-, intra- and postoperative image-based planning and review of either open or minimally invasive neurosurgical and neurological procedures. Its use is indicated for any medical condition in which the use of stereotactic surgery may be appropriate for the placement of instruments/devices and where the position of the instrument / device can be identified relative to images of the anatomy. This includes, but is not limited to, the following Cranial procedures (including frame-based stereotaxy and frame alternative-based stereotaxy):
- Lead placement and detection (DBS procedures)
iPlan's indications for use are the viewing, presentation, and documentation of medical imaging, including different modules for image processing, image fusion, atlas assisted visualization and segmentation, intraoperative functional planning where the output can be used e.g. with stereotactic image guided surgery or other devices for further processing and visualization. Example procedures include, but are not limited to:
- Planning and simulation of cranial surgical procedures such as tumor resection, shunt placement, minimal-invasive stereotactic interventions, biopsy, planning, and simulation of trajectories for stimulation and electrode recording.
Indication for Use: The Boston Scientific Vercise™ PC, Vercise Gevia™, Vercise Genus™ Deep Brain Stimulation Systems are indicated for use in:
Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
Bilateral stimulation of the internal globus pallidus (GPi) as an adjunctive therapy in reducing some of the symptoms of advanced levodopa responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
Unilateral thalamic stimulation of the ventral intermediate nucleus (VIM) is indicated for the suppression of tremor in the upper extremity. The system is intended for use in patients who are diagnosed with essential tremor or parkinsonian tremor not adequately controlled by medications and where the tremor constitutes a significant functional disability.
The Boston Scientific Vercise Deep Brain Stimulation System is indicated for use in:
- Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
Contraindications, warnings, precautions, side effects: The Deep Brain Stimulation Systems or any of its components, is contraindicated for: Diathermy as either a treatment for a medical condition or as part of a surgical procedure, Electroconvulsive Therapy (ECT) and Transcranial Magnetic Stimulation (TMS) as the safety of these therapies in patients implanted with the Vercise™ DBS System has not been established, patients who are unable to operate the system, patients who are poor surgical candidates or who experience unsuccessful test stimulation. Patients implanted with Boston Scientific Deep Brain Stimulation Systems without ImageReady™ MRI Technology should not be exposed to Magnetic Resonance Imaging (MRI). Patients implanted with Vercise Gevia™ or Vercise Genus™ or Vercise DBS Lead-only system (before Stimulator is implanted) with ImageReady MRI Technology are Full Body MR Conditional only when exposed to the MRI environment under the specific conditions defined in ImageReady MRI Guidelines for Boston Scientific Deep Brain Stimulation Systems. Assess patients for the risks of depression and suicide. This assessment should consider both the risk of depression and suicide as well as the potential clinical benefits of DBS therapy. Monitor patients for new or worsening symptoms of depression, suicidal thoughts or behaviors, or changes in mood or impulse control and manage appropriately. Refer to the Instructions for Use provided with the Vercise DBS System or BostonScientific.com for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.