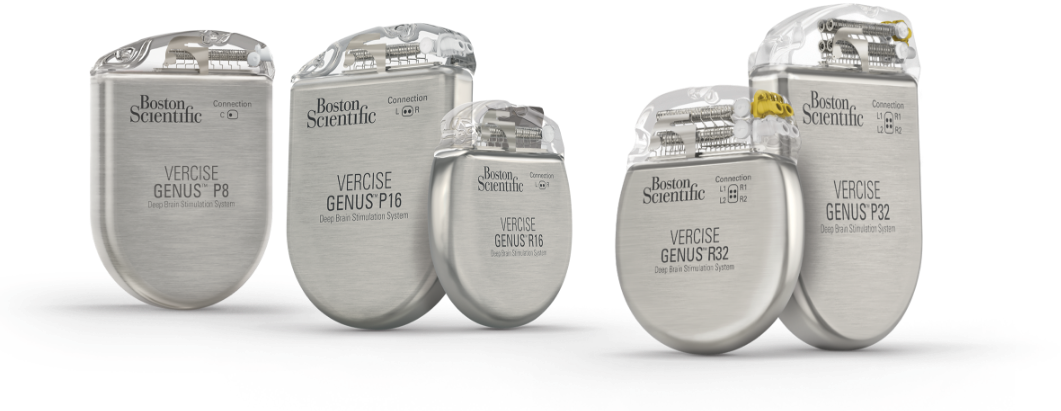
The Vercise™ M8 Adapter is a 1 x 8 in-line connector that is designed to connect specific Medtronic® lead extensions to the Boston Scientific DBS System Stimulator, as part of a deep brain stimulation procedure. The Boston Scientific Vercise M8 Adapter is compatible with the following Medtronic Leads: Model 3387 Lead, Model 3389 Lead. The Boston Scientific Vercise M8 Adapter is compatible with the following Medtronic lead extensions Model 3708640 Extension, Model 3708660 Extension, Model 3708695 Extension, Model 3708540, Model 3708560, Model 3708595 Extension.
The Vercise™ Adapter S8 is a 1 x 8 in-line connector that is designed to connect specific Abbott lead extensions to the Boston Scientific DBS System Stimulator, as part of a deep brain stimulation procedure. The Boston Scientific Vercise Adapter S8 is compatible with the following Abbott lead extensions Model 6371 Extension, Model 6372 Extension, 6373 Extension, Model 6377 Extension, Model 6378 Extension, Model 6379 Extension, Model 6170 Extension, 6172 Extension, Model 6173 Extension, Model 6178 Extension, Model 6179 Extension, Model 6180 Extension, Model 6181 Extension.
REFERENCES:
1. Boston Scientific data on file.
2. Yu X, et al. (2013). Characterizing rechargeable IPG charge cycle time in DBS. North American Neuromodulation Society (NANS) 2013.
3. Information for competitive devices accessed from https://www.medtronic.com/content/dam/emanuals/neuro/M939238A_a_060_view.pdf; accessed on Oct 5, 2023, and Boston Scientific data on file.
4. Ojukwu DI, Wang AR, Hornbeck TS, Lim EA, Sharrard J, Dhall R, Buch VP, Halpern CH. Conversion to Hybrid Deep Brain Stimulation System to Enable Multi-Contact Fractionation Can be Therapeutic. Mov Disord. 2022 Apr 7. doi: 10.1002/mds.29007. Epub ahead of print. PMID: 35393689.
INDICATION FOR USE:
The Boston Scientific Vercise™ PC, Vercise Gevia™, Vercise Genus™ Deep Brain Stimulation Systems are indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Bilateral stimulation of the internal globus pallidus (GPi) as an adjunctive therapy in reducing some of the symptoms of advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Unilateral thalamic stimulation of the ventral intermediate nucleus (VIM) is indicated for the suppression of tremor in the upper extremity. The system is intended for use in patients who are diagnosed with essential tremor or parkinsonian tremor not adequately controlled by medications and where the tremor constitutes a significant functional disability.
-Bilateral stimulation of the ventral intermediate nucleus (VIM) of the thalamus for the suppression of disabling upper extremity tremor in adult essential tremor patients whose tremor is not adequately controlled by medications and where the tremor constitutes a significant functional disability.
The Boston Scientific Vercise Deep Brain Stimulation System is indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, SIDE EFFECTS:
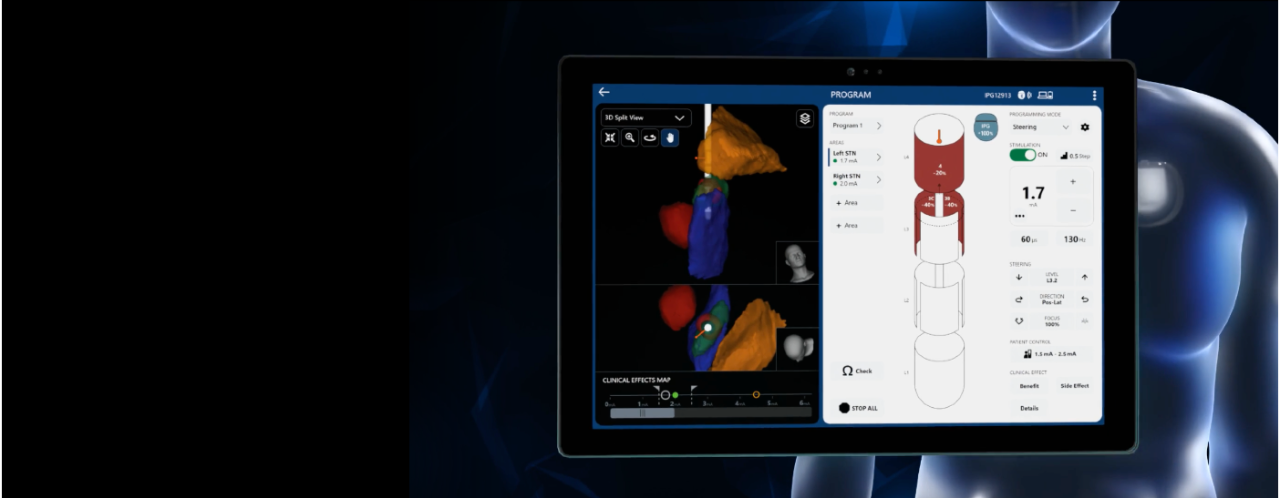
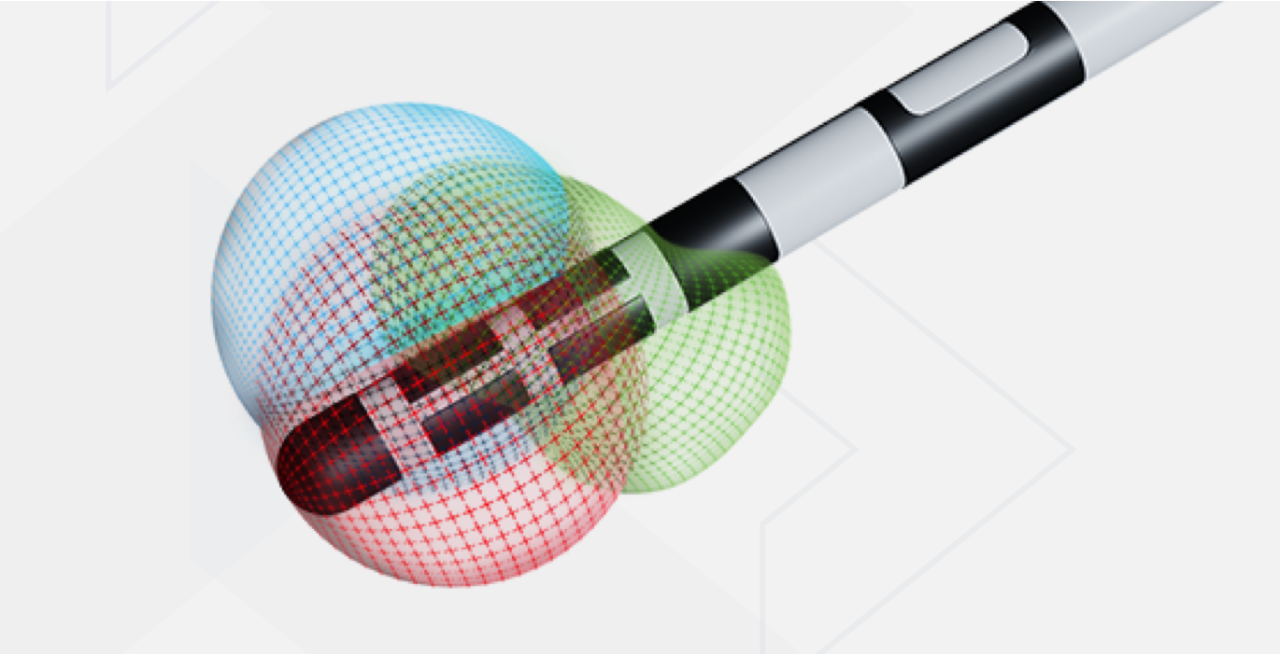
The Boston Scientific Deep Brain Stimulation (DBS) Systems or any of its components, are contraindicated for: Diathermy as either a treatment for a medical condition or as part of a surgical procedure, Electroconvulsive Therapy (ECT) and Transcranial Magnetic Stimulation (TMS) as the safety of these therapies in patients implanted with the Boston Scientific DBS System has not been established, patients who are unable to operate the system, patients who are poor surgical candidates or who experience unsuccessful test stimulation. Patients implanted with Boston Scientific DBS System without ImageReady™ MRI Technology should not be exposed to Magnetic Resonance Imaging (MRI). Patients implanted with Vercise Gevia or Vercise Genus or Vercise Genus Mixed System with M8 Adapter or Vercise DBS Lead-Only System (before Stimulator is implanted) with ImageReady MRI Technology are Full Body MR Conditional only when exposed to the MRI environment under the specific conditions defined in ImageReady MRI Guidelines for Boston Scientific DBS Systems. Assess patients for the risks of depression and suicide. This assessment should consider both the risk of depression and suicide as well as the potential clinical benefits of DBS therapy. Monitor patients for new or worsening symptoms of depression, suicidal thoughts or behaviors, or changes in mood or impulse control and manage appropriately. Refer to the Instructions for Use provided with the Boston Scientific DBS Systems or BostonScientific.com for potential adverse effects, warnings, and precautions prior to using this product. Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.