Help get HCC patients to transplant
TheraSphere™ Y-90 Glass Microspheres is the market leader and first FDA approved transarterial radiation therapy for hepatocellular carcinoma (HCC), delivering targeted damage to the tumor, sparing healthy tissue, and preserving treatment options.
TheraSphere is an efficient and powerful therapy for HCC that safely delivers high-dose radiation which can:
- Aid in achieving surgical candidacy
- Preserve liver transplant eligibility
- Support strong Overall Survival (OS) outcomes
Most utilized LRT bridging strategy for liver transplant candidates with HCC in the US¹
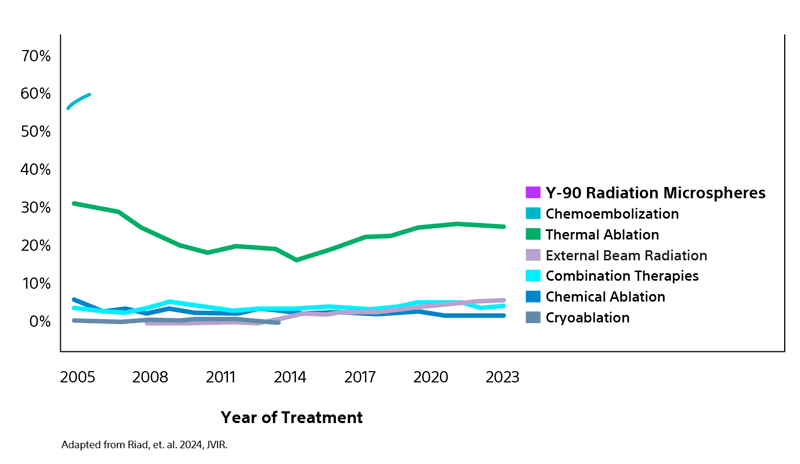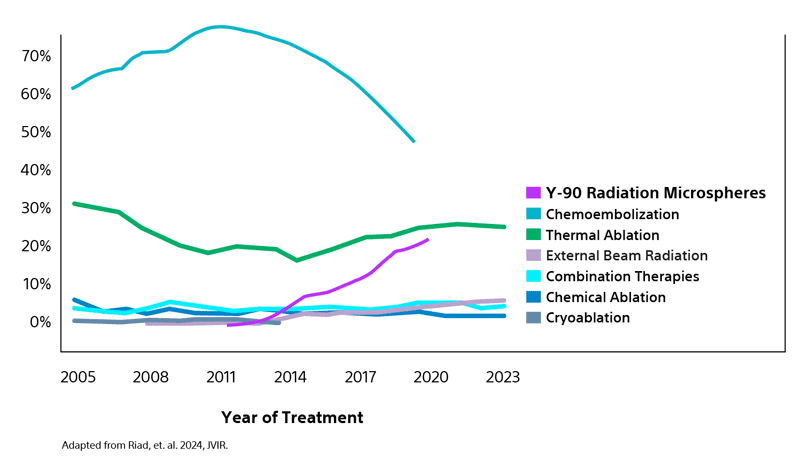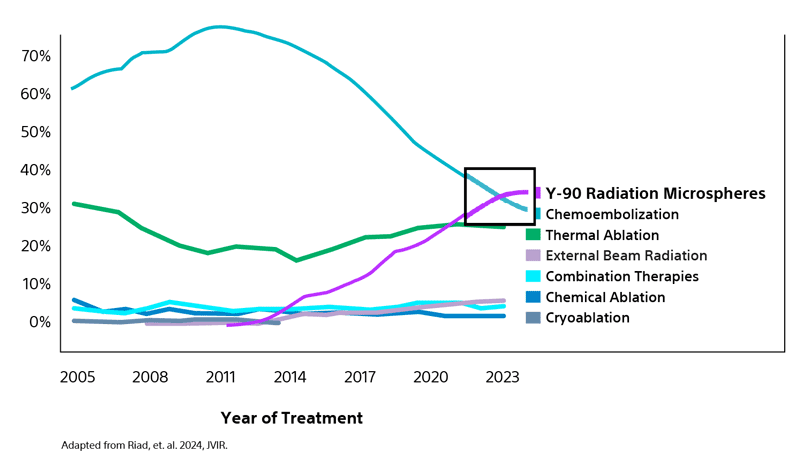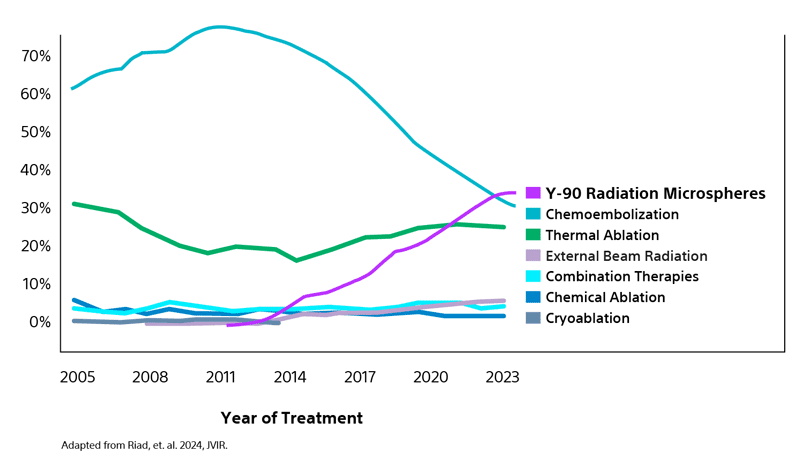
Analysis of data from the Organ Procurement and Transplant Network (OPTN) reflects the increasing adoption by many transplant centers of transarterial radioembolization (TARE) as the primary LRT option surpassing both chemoembolization and thermal ablation.¹
Bridging and Downstaging
TheraSphere provides a strong local tumor response and has been studied in patients intended to downstage tumors for transplant or bridge within Milan criteria.
