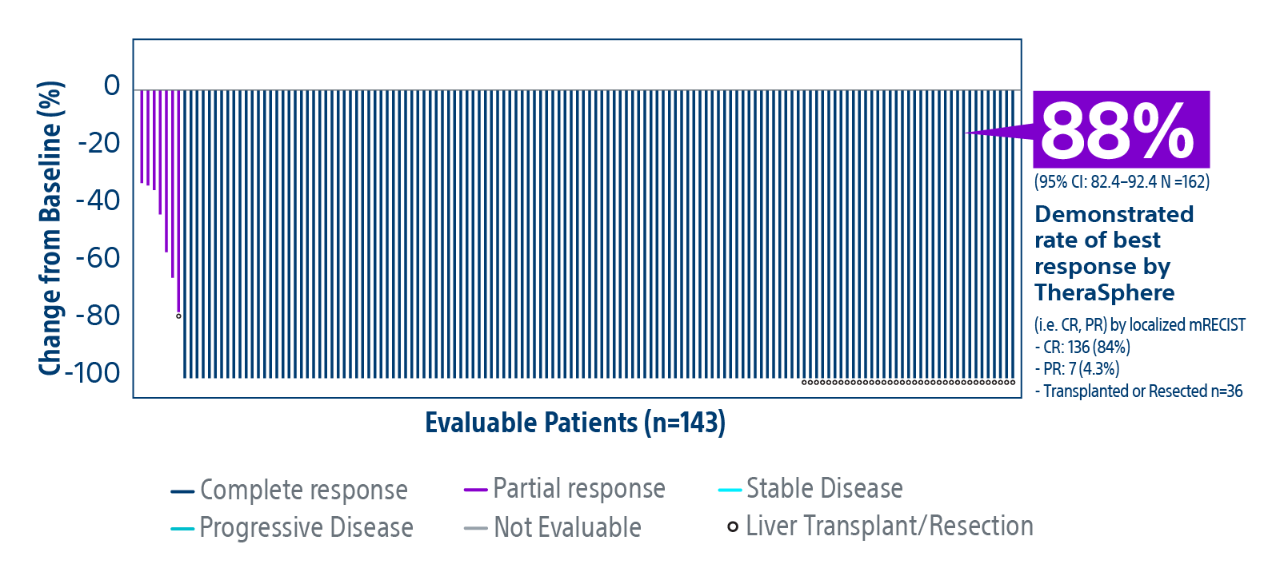
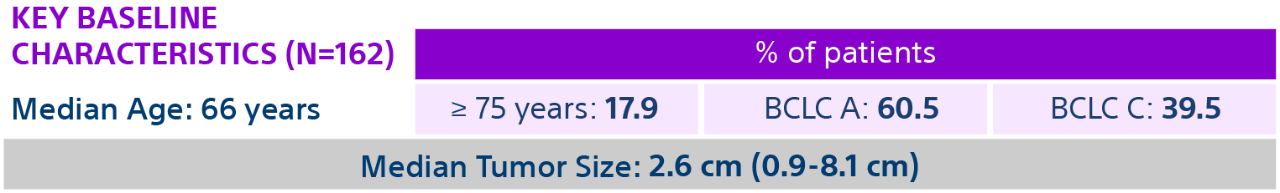
HCC: hepatocellular carcinoma; BCLC: Barcelona Clinic Liver Cancer staging system; Y-90: Yttrium-90; Gy: Gray; IQR: Interquartile Range; CR: Complete Response; PR: Partial Response.
* University of Washington, Seattle, WA; Northwestern University, Chicago, IL; Mount Sinai Health System, New York, NY.
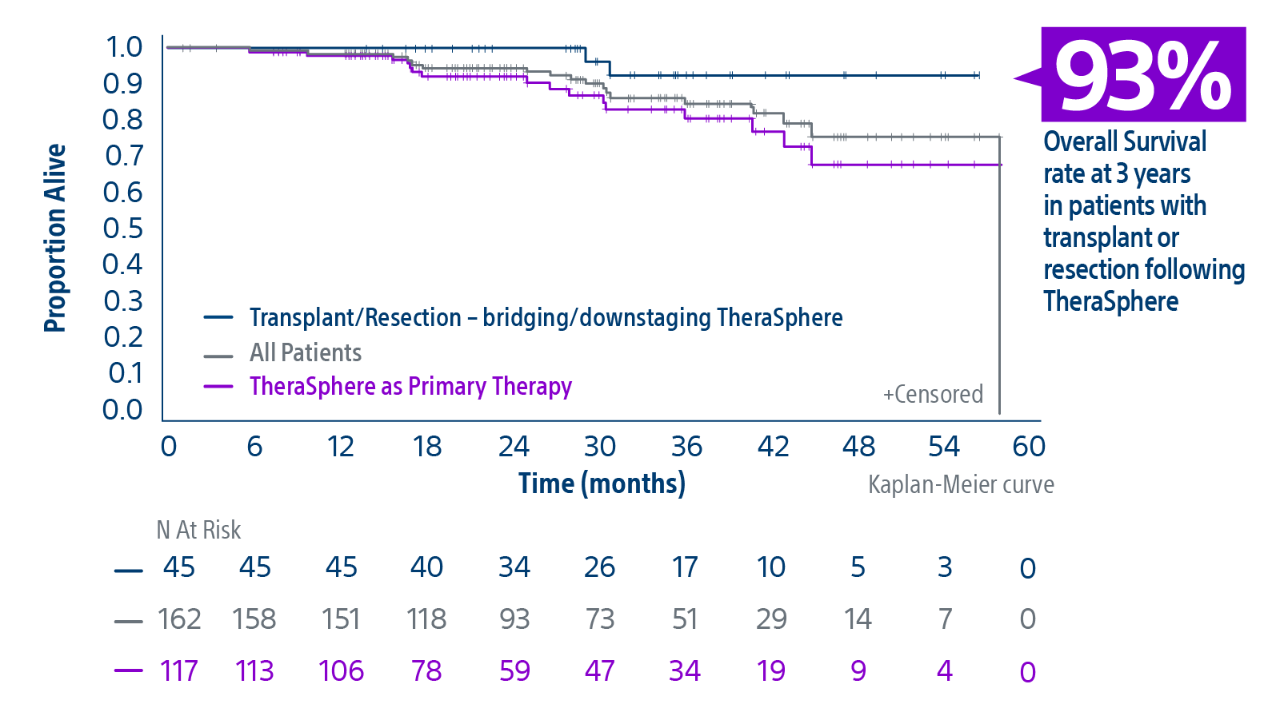
** Median follow-up was 29.9 months [95% CI: 24.7, 34.6]..
- Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, Lewandowski R, Padia SA. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable Hepatocellular Carcinoma: The LEGACY Study. Hepatology. 2021 Mar 19. doi: 10.1002/hep.31819.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2021;S0168-8278(21)02223-6. doi:10.1016/j.jhep.2021.11.018.