Boston Scientific accounts are for healthcare professionals only.

FARAPOINT™ Pulsed Field Ablation Catheter
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Ordering information
- Training
- Resources
How it works
The FARAPOINT PFA Catheter delivers focal and linear-shaped lesions with a 2.5 second application time per ablation. Fully integrated with the FARAVIEW™ Software Module and the OPAL HDx™ Mapping System, FARAPOINT offers magnetic navigation for complete visualization of catheter positioning and FIELDTAG™ Technology for confident PFA delivery.
How does FARAPOINT compare to RFA for CTI ablation?
In the ADVANTAGE AF Phase II clinical trial subanalysis, FARAPOINT demonstrated comparable safety to RFA-CTI with no instances of coronary spasm, and superior consistency with significantly lower variability in CTI applications and total procedure time.1,2
Transform focal procedures
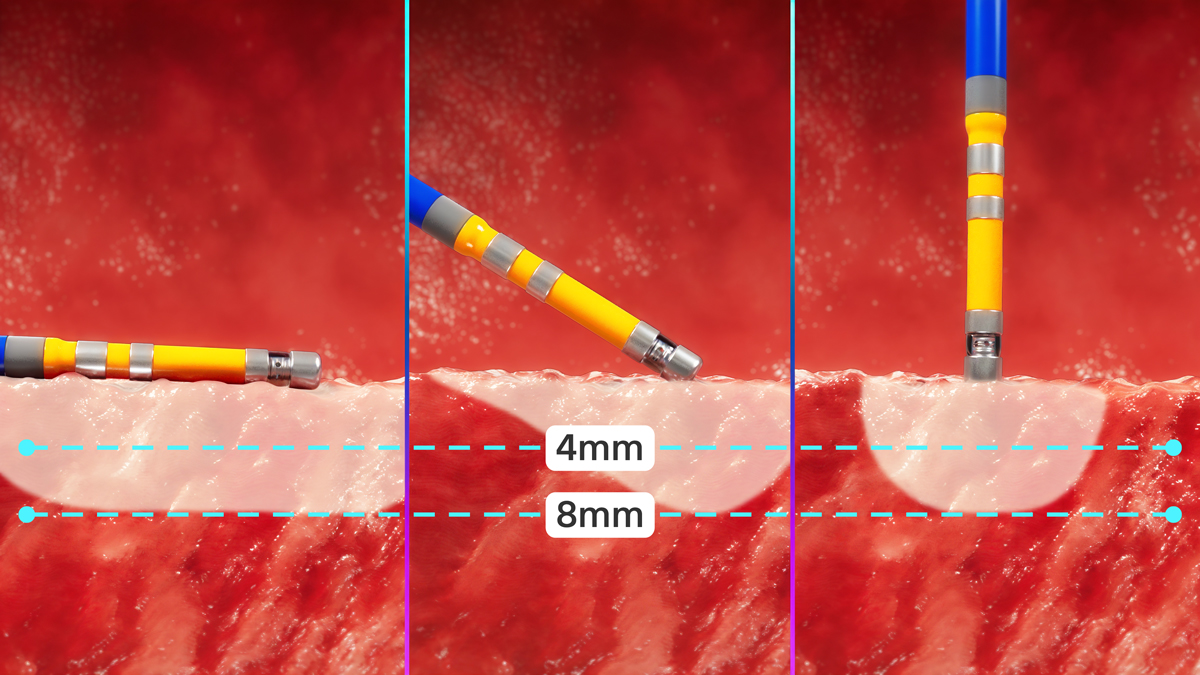
Designed for workflow versatility and point-by-point predictability, FARAPOINT creates both focal and linear-shaped lesions during ablation while maintaining consistent lesion depth.
In a preclinical porcine study, FARAPOINT delivered lesion depths up to 8.0 mm with variable voltage in multiple applications. FARAPOINT is designed to overcome challenges like ridges, pouches and variations in cardiac tissue substrates.
Real-world experience
Hear first-hand from physicians about their experience with FARAPOINT, its workflow, and its impact during the ADVANTAGE AF Phase II trial.
The benefits of purpose-built PFA
Dr. Hounshell shares insights into the benefits of the purpose-built FARAPOINT™ Pulsed Field Ablation Catheter for point-by-point workflows.
FARAPOINT CTI – ADVANTAGE AF Phase II Outcomes
Dr. Amin shares his experience using the FARAPOINT PFA Catheter during Phase II of the ADVANTAGE AF IDE Clinical Trial.

FARAPULSE
The most proven PFA platform
Discover what peers are saying about FARAPULSE
Hear firsthand from physicians as they share their excitement using the FARAPULSE PFA Platform.
Clinical highlights
ADVANTAGE AF IDE trial: Phase II CTI cohort results
The ADVANTAGE AF US IDE trial1 studied patients with persistent atrial fibrillation (PersAF) who were treated using pulmonary vein isolation (PVI) and posterior wall ablation (PWA) with the FARAWAVE™ Pulsed Field Ablation (PFA) Catheter. The trial included adjunctive ablation of the cavotricuspid isthmus (CTI) to treat typical atrial flutter (AFL).
The trial enrolled 637 patients in two phases and allowed for a comparison of CTI ablation methods:
- Phase I (n=50): CTI ablation was performed using traditional radiofrequency ablation (RFA)
- Phase II (n=141): CTI ablation was performed using the focal FARAPOINT PFA Catheter
A prophylactic nitroglycerin (NTG) protocol was implemented to reduce the risk of coronary artery spasm during FARAPOINT CTI procedures.
96.4%
[97.5% LCL = 91.7%]
CTI effectiveness event-free rate at 12 months
0
No reports of coronary spasm (no ST segment elevation or ventricular fibrillation)
CTI Effectiveness Event-Free Rate
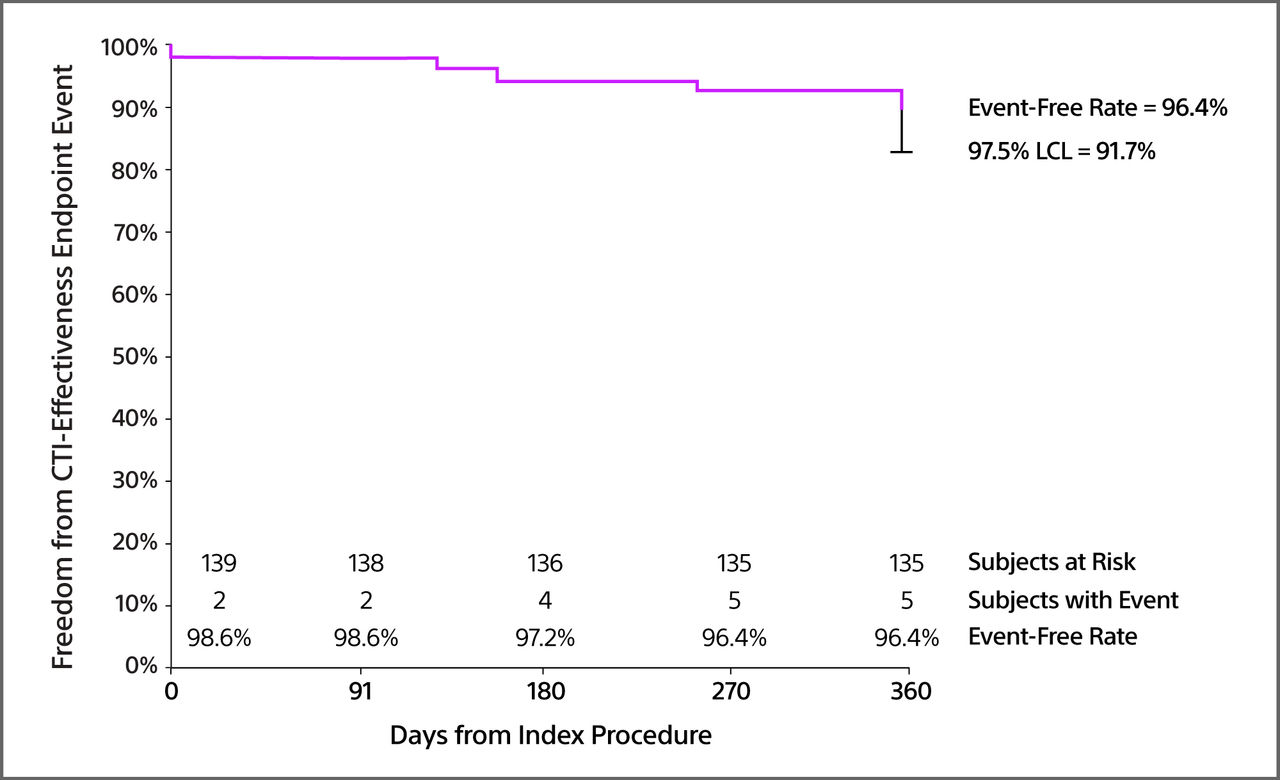
CTI effectiveness endpoint: The composite CTI effectiveness endpoint (acute bidirectional block and 1-year freedom from documented CTI-dependent AFL ≥ 10 sec on ECG without repeat CTI ablation) was achieved in 96.4% of the cohort.
Sub-analysis: CTI Phase I (RFA) vs CTI Phase II (PFA/FARAPOINT)2
FARAPOINT procedures were more predictable, had shorter fluoroscopy times, and significantly reduced variability in the number of CTI applications†.
| Phase I (RFA-CTI) | Phase II (PFA-CTI) FARAPOINT | |
| Patient # receiving CTI | 50 (19.2%) | 141 (55.3%) |
| CTI applications | 22.5 ± 18 | 17.5 ± 6.4 |
| Procedure time (min) | 125.3 ± 45.3 | 105.9 ± 34.9 |
| Dwell time (min) | 58.9 ± 20.2 | 59.1 ± 23.6 |
| Fluoroscopy time (min) | 24.6 ± 15.2 | 16.4 ± 12.3 |
| CTI Ablation time (min) | Not Collected | 8.4 ± 13 |
Data in table reported as Mean ± SD or n (%) as appropriate.
† Variance of number of CTI applications showed significantly lower variability with FARAPOINT-CTI vs RFA-CTI (F=8.0, p=0.001).
The focal FARAPOINT PFA Catheter provided comparable freedom from atrial flutter recurrence and safety outcomes.
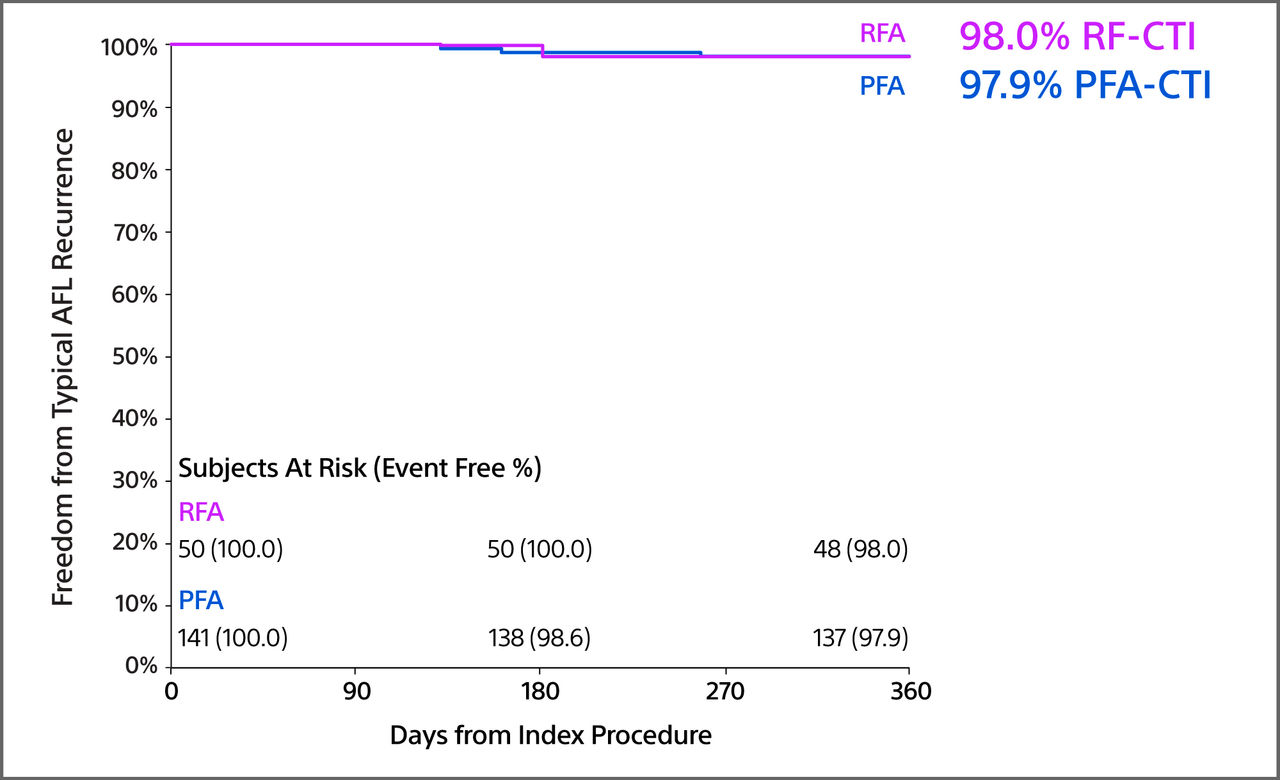
Freedom from Typical AFL Recurrence
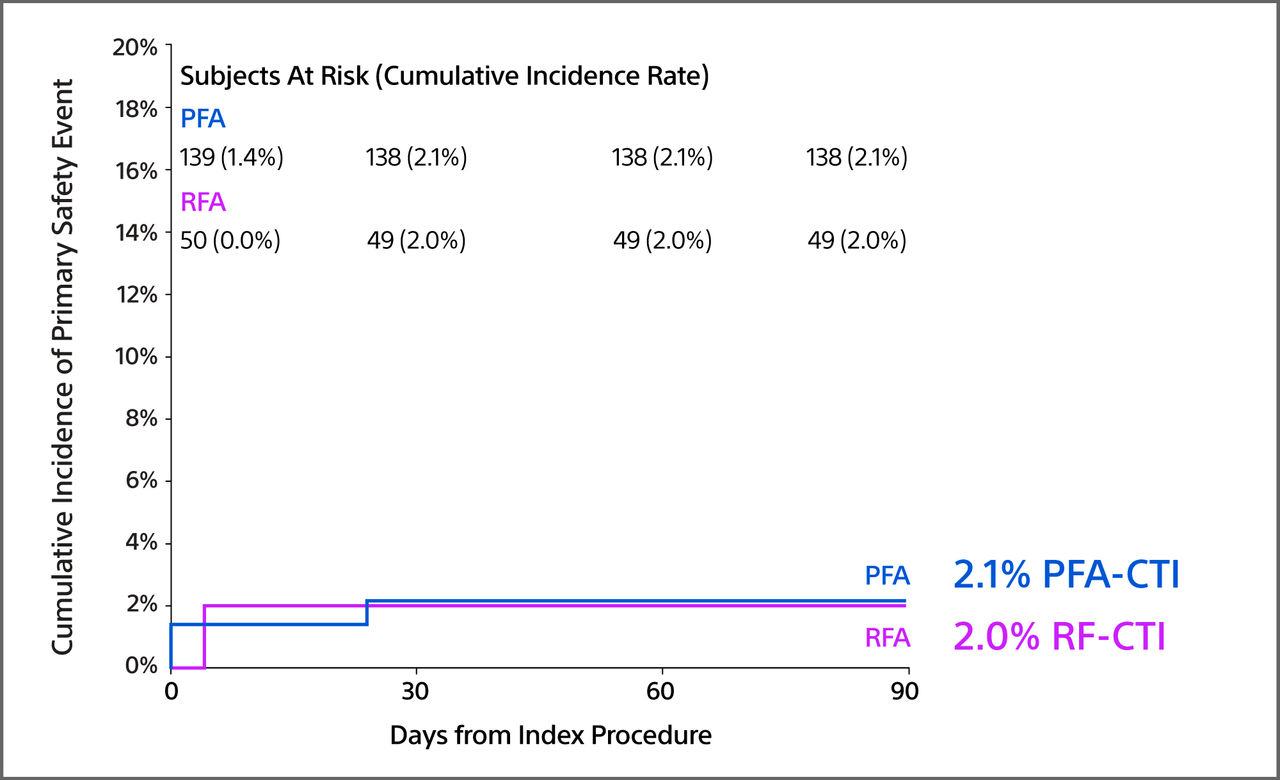
CTI Primary Safety Event Rate
Sub-analysis conclusions
- Safety rates were similar (PFA=2.1% vs RFA=2.0%; p=NS). There were no instances of clinical coronary artery spasm in those who were treated with FARAPOINT (99.0% received NTG per protocol with 54.0% receiving only 3 mg).
- The freedom from recurrence of typical AFL was similar in the two cohorts at 97.9% for FARAPOINT-CTI and 98.0% for RFA-CTI.
- Significantly lower variability with FARAPOINT PFA-CTI vs RFA-CTI in number of applications and total procedure time (CTI applications F=8.0, p=0.001 and total procedure time F=1.7, p=0.02)
Discover what peers are saying about FARAPULSE
Hear firsthand from physicians as they share their experience using the FARAPULSE PFA Platform
FARAPOINT PFA Catheter technical specifications
| Feature | Catheter specifications |
| Catheter type | Focal |
| Energy modality | Bipolar, biphasic PFA |
| Tip diameter | 2.5 mm |
| Shaft diameter | 8F |
| Usable length | 115 cm |
| Number of electrodes | 4 |
| Electrode spacing | 1.5 mm – 6.0 mm – 1.5 mm |
| Electrode sizing | 2.0 mm – 1.5 mm – 1.5 mm – 2.0 mm |
| Sheath compatibility | Any 8.5F Sheath |
Boston Scientific Compatible: FARADRIVE™ Steerable Sheath (M004PF21M402) | |
| VersaCross™ integration | VersaCross Connect™ Access Solution for FARADRIVE™ VersaCross™ Steerable Access Solution |
FARAPOINT PFA Catheter ordering information
| Product description | Reference number |
| FARAPOINT PFA Catheter (D-F Curve) | M004PF81M110 |
| FARAPOINT PFA Catheter (F-F Curve) | M004PF81M310 |
Accessories
| Product description | Reference number |
| PFA Gen 2 Connection Cable for FARAPOINT | M004PF41M444 |
Online education
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
FARAPOINT clinical data
Additional FARAPULSE PFA Platform resources
Find FARAPULSE™ PFA Platform product brochures, spec sheets, clinical data compilations and more on our FARAPULSE resources page.
Required products
†CAUTION: FARAWAVE™ NAV PFA Catheter was not used in this study.
*Baylis Medical Company Radiofrequency Puncture Generator RFP-100A. Baylis Medical Company is a wholly owned subsidiary of Boston Scientific Corporation.
References:
1. Reddy, V., et al., "Pulsed Field Ablation of Persistent Atrial Fibrillation With Continuous ECG Monitoring Follow-Up: ADVANTAGE AF-Phase 2." Circulation 151.0 (2025).
2. Reddy, VY, et al., 2025. Focal Pulsed Field Ablation vs Standard Radiofrequency Ablation for Typical Atrial Flutter: A sub-study across Phase I and Phase II of the Pivotal ADVANTAGE AF Trial. Presented at HRS.