Boston Scientific accounts are for healthcare professionals only.

FARAWAVE™ Pulsed Field Ablation Catheter
Configure or select a product to continue to order
- Overview
- Technical specifications
- Ordering information
- Training
- Resources
How it works
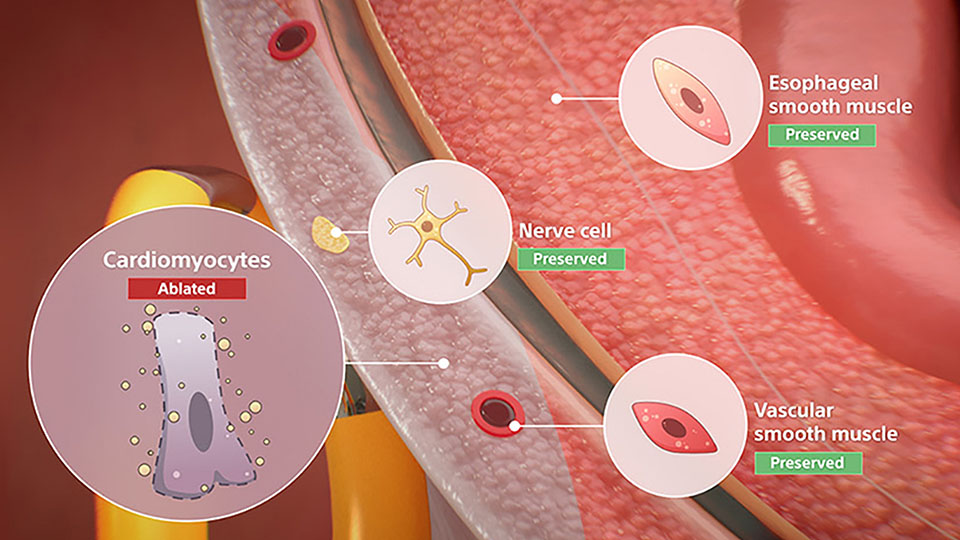
FARAWAVE delivers a proprietary waveform to preferentially ablate cardiomyocytes, which have lower thresholds to electric field damage than surrounding tissue—treating atrial fibrillation (AFib) effectively while minimizing risk of thermal complications.
Why choose FARAWAVE Pulsed Field Ablation Catheter
Optimized catheter design
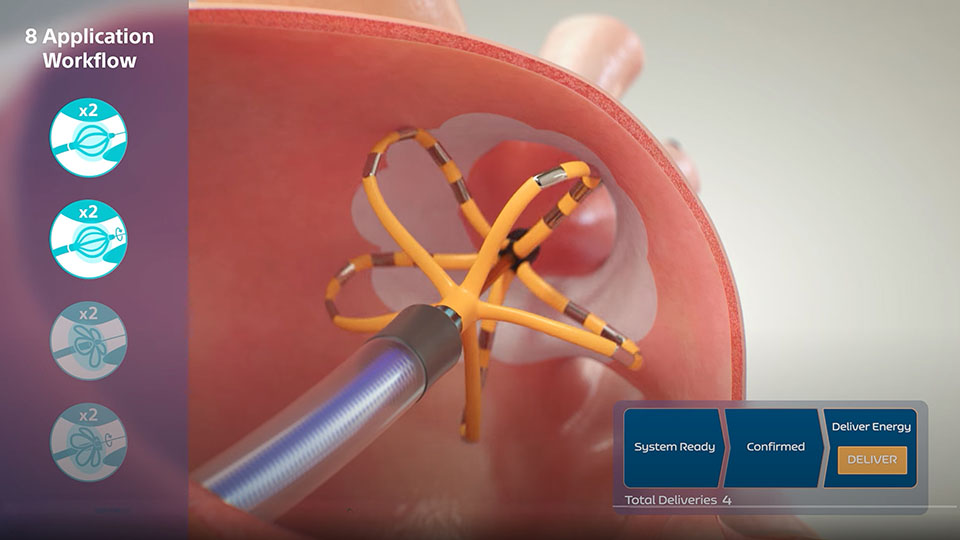
The FARAWAVE PFA Catheter has been optimized to achieve catheter placement in a wide range of pulmonary vein (PV) and posterior wall (PW) anatomies to address PFA energy delivery needs without the need to manually manage electrode activation.
Featuring two different form factors (basket and flower positions), the FARAWAVE PFA Catheter is purposefully designed to optimize:
- Maneuverability
- Placement in variable-anatomy veins
- PV and PW application
- Standardized electrode activation (manual electrode activation is not required)


FARAPULSE
The most proven PFA platform
Discover what peers are saying about FARAPULSE
Hear firsthand from physicians as they share their excitement about the
transformative FARAPULSE experience.
Required products
FARAWAVE PFA Catheter technical specifications
| Feature | Specifications |
| Catheter type | Over-the-wire |
| Shaft diameter | 12F |
| Distal diameter | 31 mm; 35 mm |
| Usable length | 115 cm |
| Number of electrodes | 20 |
| Guidewire compatibility | 0.035” diameter, 180 cm minimum length |
| Sheath compatibility | FARADRIVE Steerable Sheath, 74 cm length REF M004PF21M402 |
FARAWAVE PFA Catheter ordering information
| Product description | Reference number |
| FARAWAVE PFA Catheter 31 mm (distal diameter) | M004PF41M401 |
| FARAWAVE PFA Catheter 35 mm (distal diameter) | M004PF41M402 |
Accessories
| Product description | Reference number |
| FARASTAR catheter connection cable for FARAWAVE | M004PF41M434 |
| Merit inqWire | IQ35F180J1O5RS |
Online education
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Product specifications
Brochures
Additional FARAPULSE PFA Platform resources
Find product brochures, spec sheets, clinical data compilations and more on our FARAPULSE resources page.
* Baylis Medical Company Radiofrequency Puncture Generator RFP-100A. Baylis Medical Company is a wholly owned subsidiary of Boston Scientific Corporation.
References:
1. Reddy VY, Gerstenfeld EP, Natale A, et al., Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. New England Journal of Medicine. 2023;Nov2;389(18):1660-1671. doi:10.1056/NEJMoa2307291
2. Ekanem, E., Neuzil, P., Reichlin, T. et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat Med (2024). https://doi.org/10.1038/s41591-024-03114-3
3. Della Rocca DG, Marcon L, Magnocavallo M, et al., Pulsed electric field, cryoballoon, and radiofrequency for paroxysmal atrial fibrillation ablation: a propensity score-matched comparison, EP Europace, 2024;Jan26(1) euae016. doi.org/10.1093/europace/euae016
4. Watanabe, Keita, et al., Lesion Morphometry of the Pentaspline Catheter: Understanding Catheter Pose, Rotation, and Dosing. Circ Arrhythm Electrophysiol. 2024;17(12):e013208.