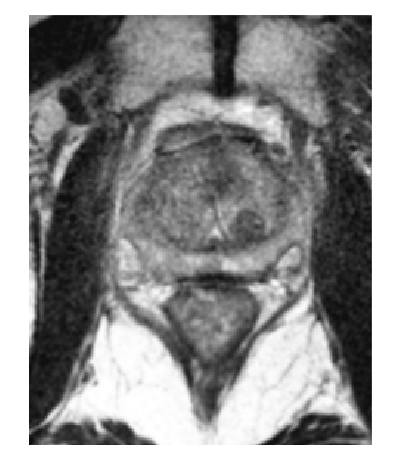
How SpaceOAR Hydrogel works
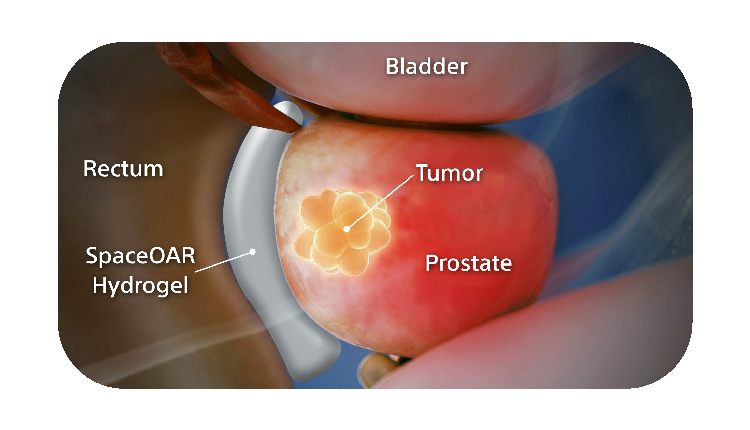
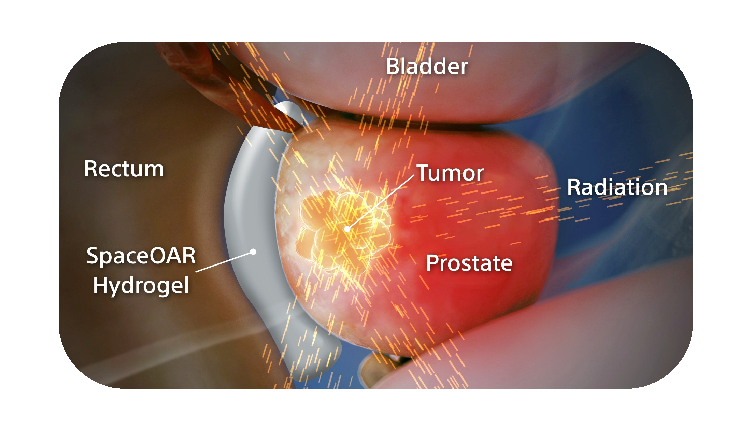
SpaceOAR Hydrogel is a PEG-based hydrogel designed to help reduce the radiation dose delivered to the rectum during prostate cancer radiation treatments.
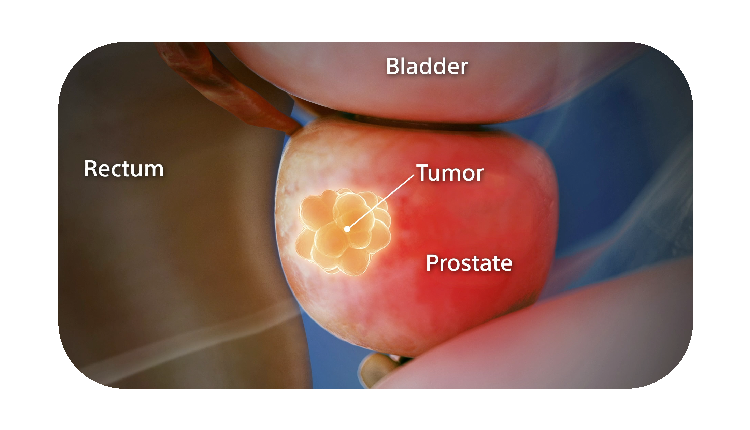
When treating prostate cancer patients with radiation therapy, the goal is to destroy the cancer cells while avoiding damage to the surrounding healthy tissue.