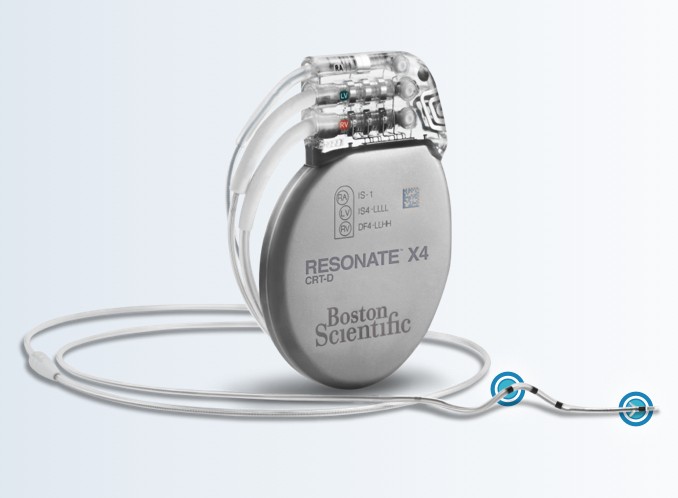
RESONATE™ HF CRT-D and
RESONATE™ X4 CRT-D
Cardiac Resynchronization Therapy Defibrillator
Model G547 and Model G447
Innovative technology, powerful insights.
Comprehensive, novel technology in our RESONATE CRT-Ds helps physicians to identify heart failure early, restore chronotrophic competence, and improve CRT response in most patients. And they are fueled by the longest-lasting battery technology available, substantially reducing major complications and costs associated with battery replacement.1In addition, comprehensive shock features to prevent sudden cardiac death are available today and for the future.
Key Resources
Explore
Product Details
Identify heart failure decompensation, increase CRT response, reduce costs, and improve quality of life in heart failure patients with our RESONATE family of implantable CRT-Ds (cardiac resynchronization therapy defibrillators).
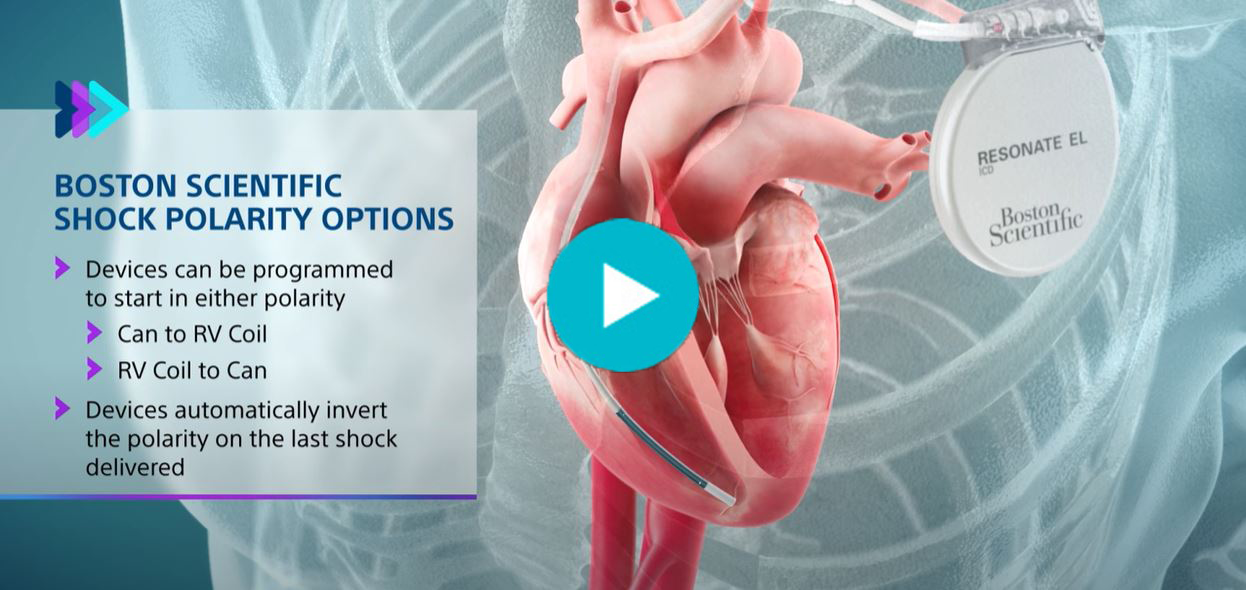
- Boston Scientific devices are unique by providing 8 shocks in the VF zone, the option to choose shock polarity, and automatically invert shock polarity in a series of shocks
- HeartLogic™ Heart Failure Diagnostic can predict heart failure events weeks in advance with minimal alerts2
- SmartCRT™ Technology provides customized programming to obtain CRT response in most patients3
- EnduraLife™ Battery Technology extends device longevity (up to 13.2 years even with MultiSite Pacing turned on)*
- RightRate™ Minute Ventilation is the only sensor clinically proven to restore chronotropic competence4 (available only in RESONATE HF)

Clinical Data
MANAGE-HF trial
Investigated the clinical integration and safety of the FDA-approved HeartLogicTM Heart Failure Diagnostic in the management of patients with heart failure9:
- HeartLogic was safely integrated into clinical care
- Early treatment augmentation was associated with more rapid recovery of the HeartLogic index compared to patients with no change in treatment following an alert
- 67% reduction in heart failure hospitalizations compared to pre-study9
SMART-MSP trial
The SMART-MSP clinical trial exceeded both its end points:
- Safety Endpoint: The MultiSite Pacing (MSP) feature-related complication-free rate at 180 days post MSP on is 99%
- Effectiveness Endpoint: 51% of the non-responders at 6 months converted to responders at 12 months3
NAVIGATE X4 trial
The NAVIGATE X4 trial was a prospective, non-randomized, multi-center, single-arm, clinical study. With 764 patients in 88 implant centers.
- 50% of ACUITY X4 leads were placed in 6 minutes or less and required no reoperations due to pacing capture or thresholds
- 77.3% of ACUITY X4 Spiral leads were programmed with a proximal electrode as the pacing cathode5
Preventing sudden cardiac death
Storm Aborted by Automated Shock Polarity Reversal in ICD
Read the HeartRhythm Case Reports article: Storm Aborted by Automated Shock Polarity Reversal in ICD.
More shocks when conversion is difficult
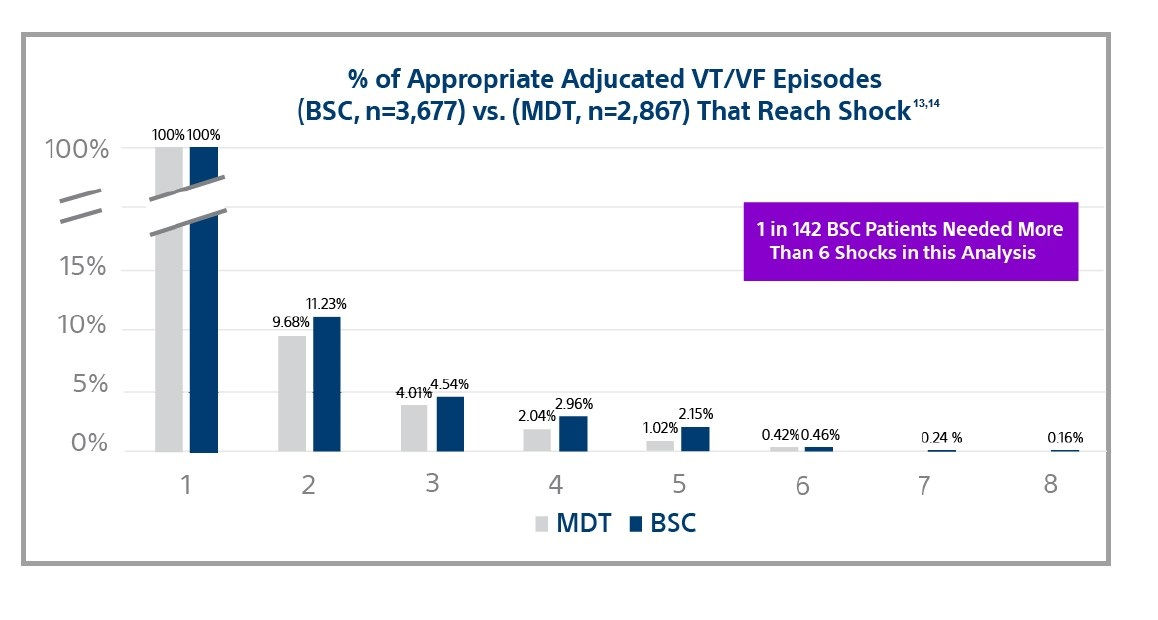
Large clinical trials show the mortality benefit of ICDs is a result of successful shocks.15,16
Among leading manufacturers, shock efficacy is nearly identical for shocks 1-6 with .46% and .42% of adjudicated VT/VF episodes reaching the 6th shock.13,14
However, Boston Scientific devices provide 8 shocks in the VF zone while others only provide 6.
Why is this important? One in 142 patients needed more than 6 shocks to convert anepisode of VT/VF.13
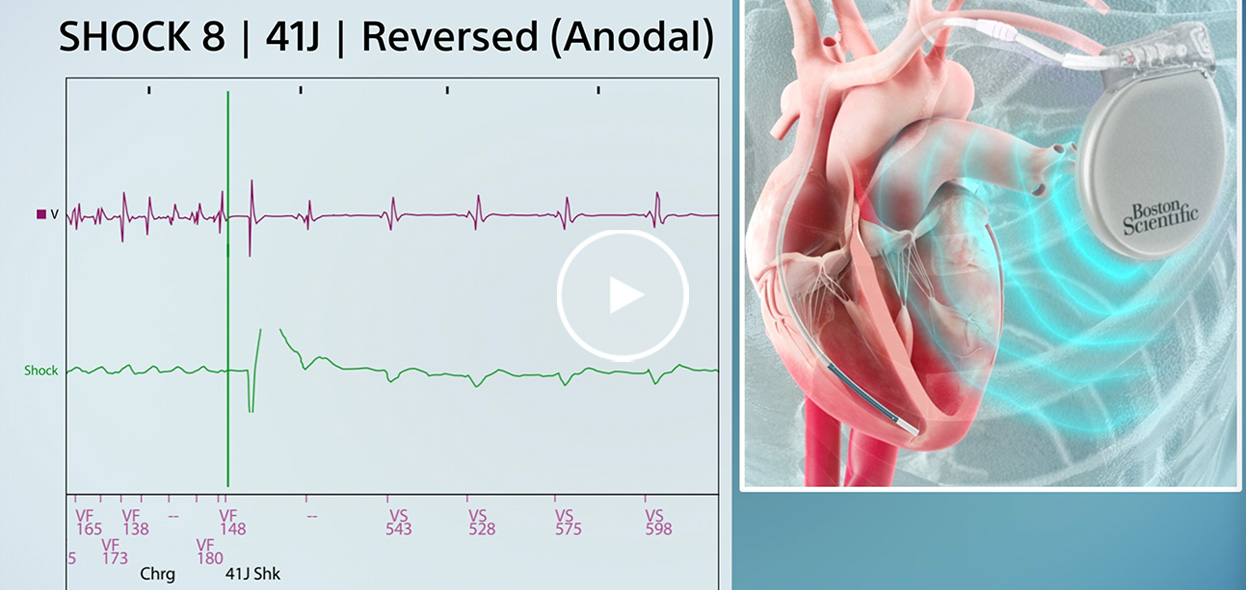
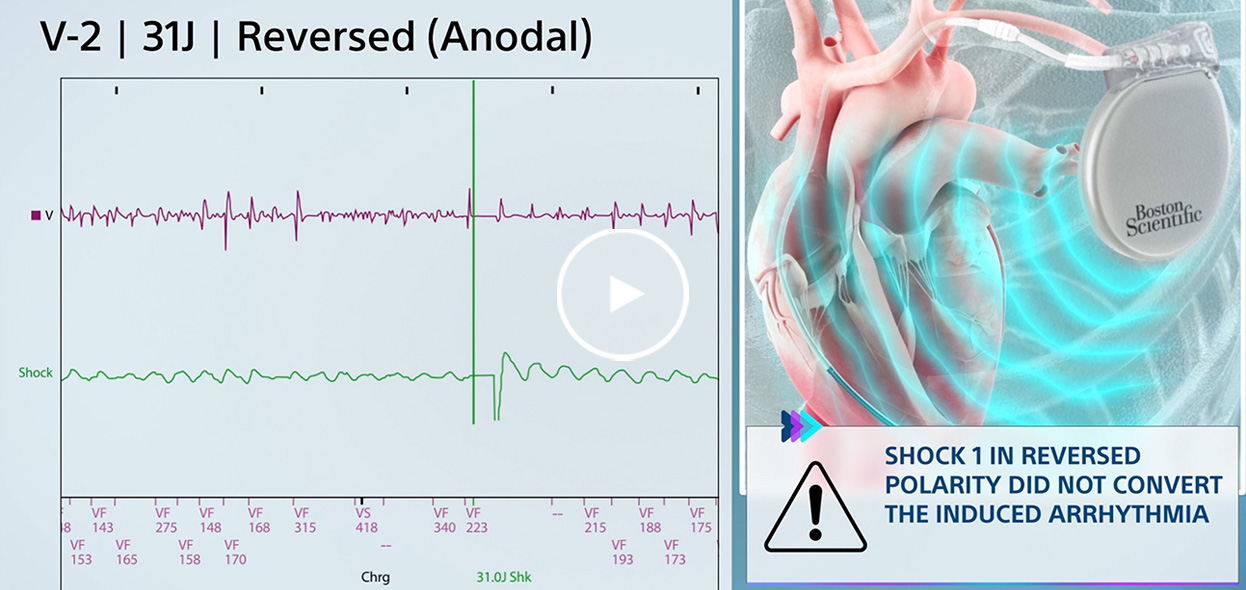
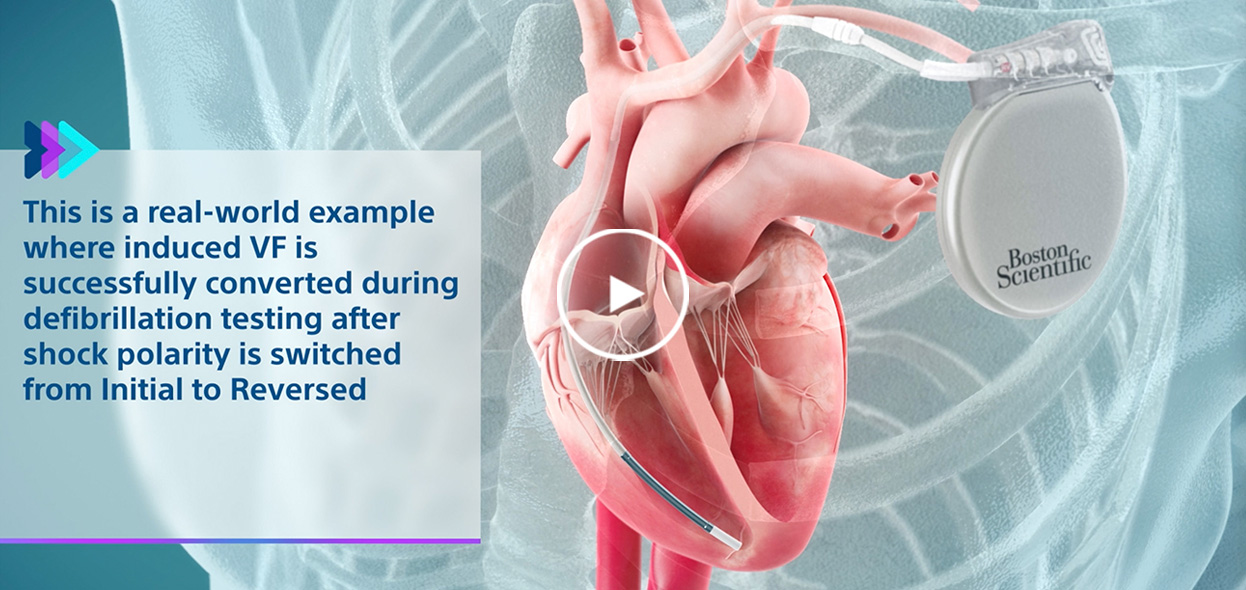
Real-world examples
See the value of Boston Scientific features to prevent sudden cardiac death in these real-world examples.
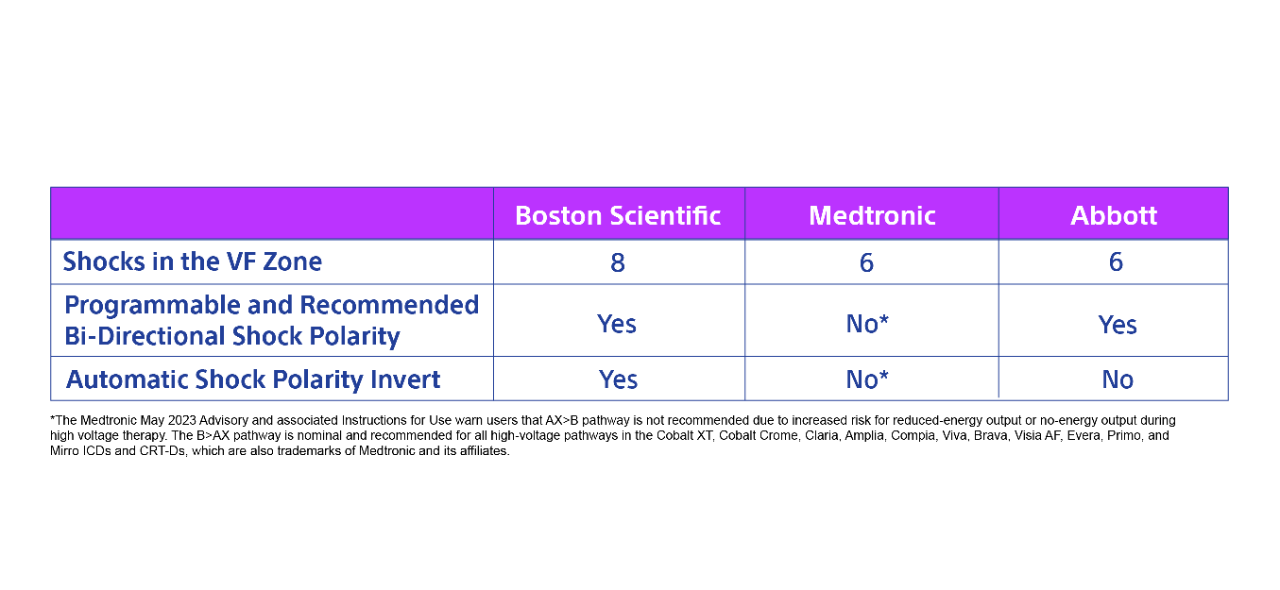
How we compare to our competitors
Boston Scientific
CRT-Ds still in service6
Medtronic
Viva™ XT CRT-Ds7 still in service
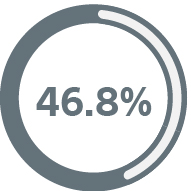
Medtronic
Viva Quad XT CRT-D8
Key features
RESONATE X4 and RESONATE HF are Boston Scientific’s most technically advanced CRT-Ds available with the following features:
HeartLogic Heart Failure Diagnostic, SmartCRT Technology, EnduraLife Battery Technology, ImageReady MR-Conditional Systems, and RightRate Minute Ventilation (available only with RESONATE HF).
HeartLogic Heart Failure Diagnostic
Predict potential heart failure events weeks in advance with HeartLogic.
Personalize cardiac care using the patient’s response to arrhythmic and pacing changes. Studies with HeartLogic devices have resulted in 67% reduction in heart failure related hospitalizations.9
SmartCRT Technology
Personalize CRT therapy to each patient for the most optimized outcomes.
SmartCRT helps you maximize patient response to CRT therapy by optimizing where, when, and how to pace.
RightRate Minute Ventilation
The only sensor clinically proven to restore chronotropic competence and maintain health heart rate variability.10
RightRate dual sensor adapts to changes in movement and respiration, regulating the patient's ability to increase their heart rate during exercise or other everyday activities. Hear directly from Don Hopper, an active patient, as he shares his journey with RESONATE HF in the video on the left.
Available only with RESONATE HF CRT-D.
EnduraLife Battery Technology
Industry-leading longevity without compromising patient care.
CRT-D devices with EnduraLife have up to 13.3 years projected longevity*, giving you the clinical freedom to make programming decisions that optimize therapy for the patient, not the device.
Fewer major complications. Better for patients.
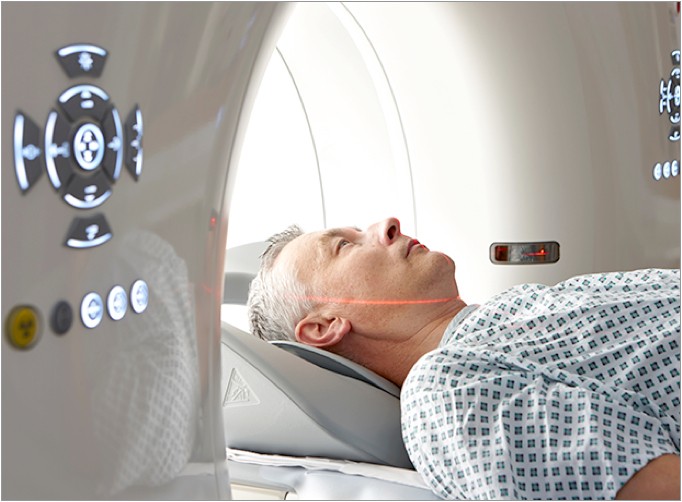
ImageReady MR-Conditional Systems
ImageReady MR-Conditional systems make MRIs possible for heart failure patients.†
Patients can safely get Full Body MRI scans at 1.5T or 3T with ImageReady MR-Conditional devices without exclusion zone, scan duration, or patient height restrictions.11,12
ImageReady is compatible with the RESONATE X4 and RESONATE HF CRT-Ds.
Product specifications
| Model | Type | Size (cm) (W x H x D) | Mass (g) | Volume (cc) | Connector Type (RA RV LV) |
|---|---|---|---|---|---|
| G547 (HF) | X4 CRT-D | 5.37 x 8.18 x 0.99 | 73.8 | 32.5 | RA: IS-1; RV: DF4; LV: IS4 |
| G447 | X4 CRT-D | 5.37 x 8.18 x 0.99 | 73.8 | 32.5 | RA: IS-1; RV: DF4; LV: IS4 |
Longevity Information
The following tables represent sample pulse generator life expectancy estimation (implant to explant) with EnduraLife batteries as provided in product labeling. For specific programmable parameter ranges, refer to product labeling at www.bostonscientific-elabeling.com, or contact Boston Scientific technical services or your local representative.
| Projected longevitya | Ventricular Chambers |
RA/RV | LV | LVbd | 500Ω with LATITUDE™b | 700Ω with LATITUDE™b |
700Ω no LATITUDE™, RS, or HFSSc |
|---|---|---|---|---|---|---|---|
| MultiSite Pacing Off | |||||||
| Typical programmed setting | BiV | 2.5 V | 3.0 V | Off | 9.7 | 10.5 | 11.3 |
| Maximum labeled longevity | LV-Only | 2.0 V / Off | 2.0 V | Off | 12.9 | 13.2 | 14.7 |
| MultiSite Pacing On | |||||||
| Typical programmed setting | BiV MSP | 2.5 V | 3.0 V | 3.0 V | 8.2 | 9.1 | 9.7 |
| Maximum labeled longevity | LV-Only MSP | 2.0 V / Off | 2.0 V | 2.0 V | 11.5 | 12.1 | 13.3 |
- Assumes 70 PPM LRL; DDDR mode; 0.4 ms Pulse Width (RA, RV, LV); sensors On, Heart Failure Sensor Suite On.
- Projected longevity is calculated assuming 2 maximum energy charging cycles per year, including automatic capacitor reforms and therapeutic shocks. These calculations also assume 3-channel EGM Onset is on and that the pulse generator spends 3 months in Storage mode during shipping and storage.
a. Assumes ZIP telemetry use for 2 hours at implant and for 40 minutes annually for in-clinic follow-up checks.
b. Assumes standard use of the LATITUDE™ Communicator as follows: Daily Device Check on, quarterly scheduled remote follow ups, and other typical interrogations.
c. Assumes LATITUDE™ Communicator is not used, Respiratory Sensor is Off, and Heart Failure Sensor Suite is Off.
d. Applies to models with MultiSite Pacing (MSP).
Additional Longevity Information
- Boston Scientific devices have corporate warranties at 6 years in available geographies. Warranty information available at www.bostonscientific.com/warranty.
- Devices use Li/MnO2 chemistry.
- The Usable Battery Capacity is 1.9 Amp-hours (typical implant to battery capacity depleted).
- Shelf life is 2 years (before use by date).
Ordering information
| Model | Description |
|---|---|
| G547 | RESONATE™ HF CRT-D |
| G447 | RESONATE™ X4 CRT-D |
Reimbursement
Education and training
Educational Resources
Explore CRT-D training and resources, best practices modules, medical training, and continuing education courses.