Boston Scientific is no longer seeking regulatory approval of ACURATE neo2™ or ACURATE Prime™ in the United States or other countries. This decision was made due to the increased clinical and regulatory requirements to maintain regulatory approvals in global markets and to obtain approvals in new regions.
Patients who have been treated with an ACURATE neo2 valve or ACURATE Prime XL valve as part of the ACURATE IDE clinical trial should continue with their study follow-up care.
For Investigators that would like more information, please contact the Boston Scientific ACURATE IDE study team.
The ACURATE™ Aortic Valve Platform
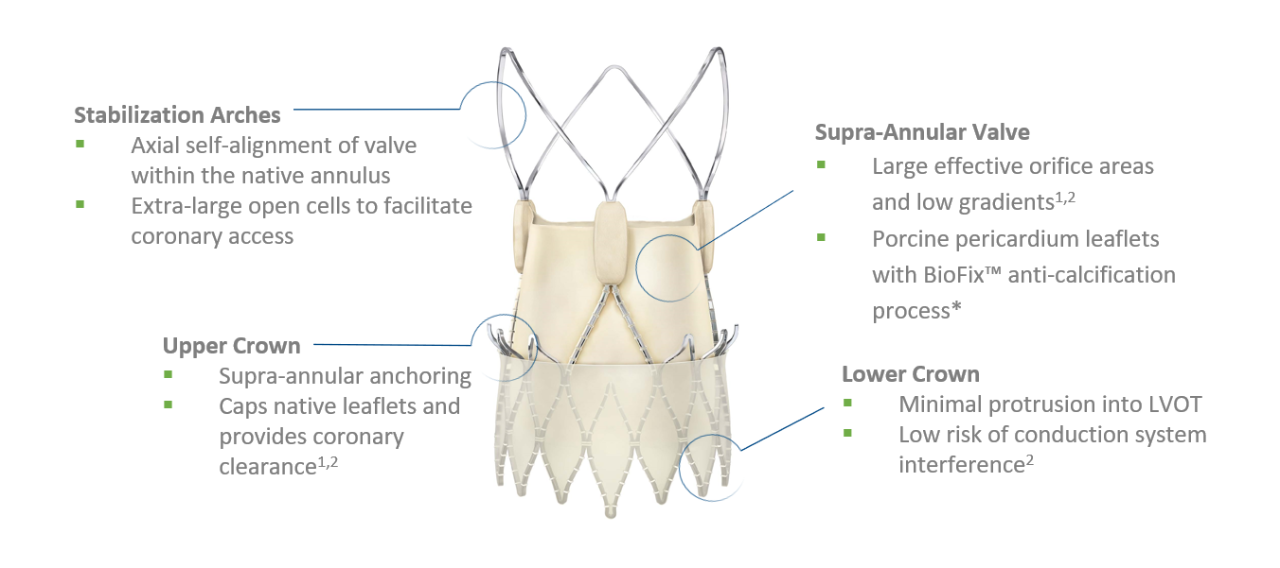
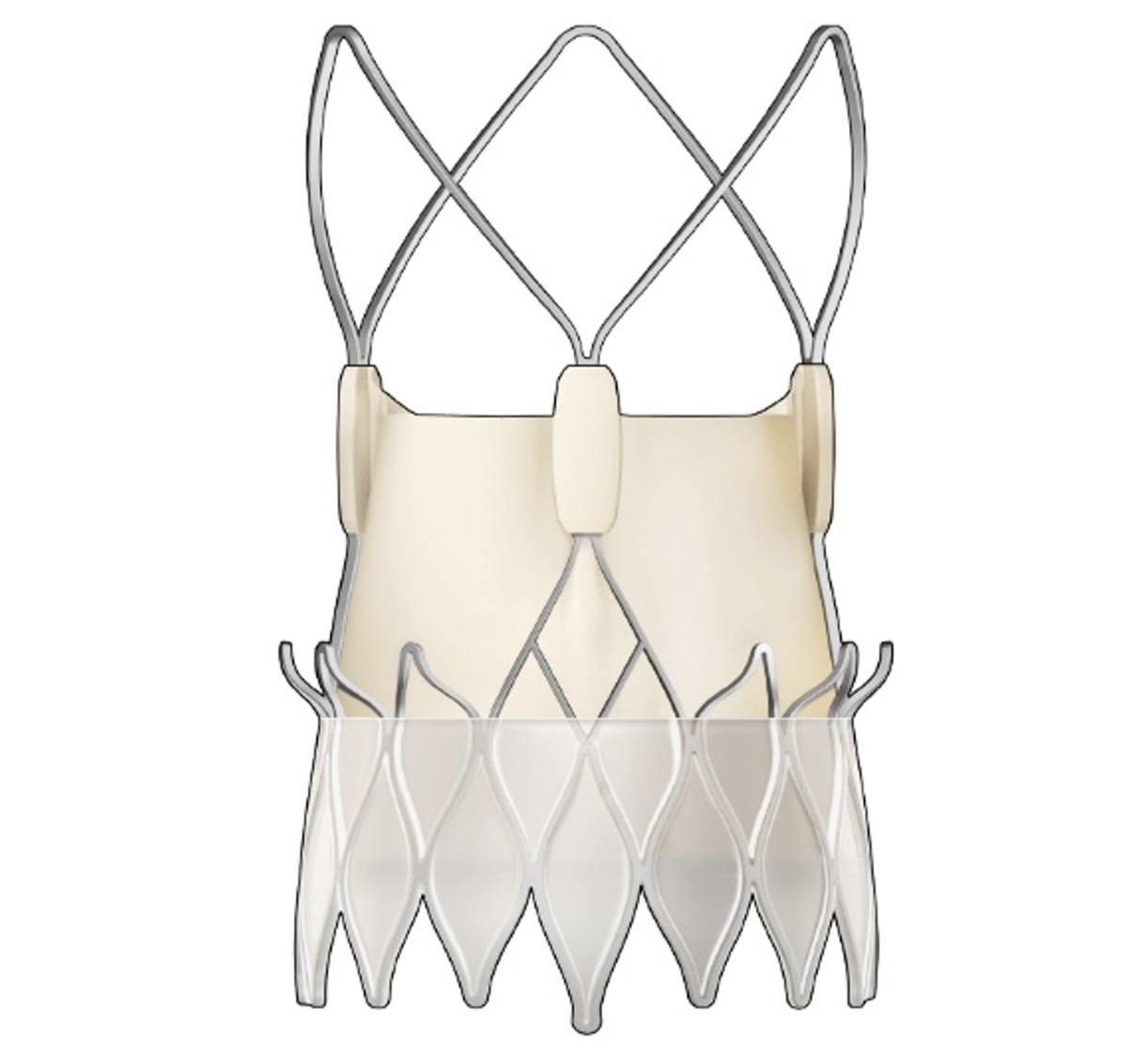
The ACURATE neo2™ Aortic Valve System is the next iteration of the ACURATE neo™ Aortic Valve System. It features Active PVseal™ technology and an extended sealing skirt to cover the full waist of the stent, a radiopaque positioning marker designed to give a clear visual reference for easy and accurate positioning, and extra wide stabilization arches to facilitate coronary access. The ACURATE neo2 Valve received CE mark in April 2020.
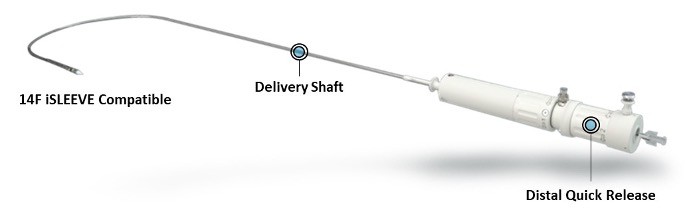
ACURATE Prime Transfemoral (TF) Delivery System XL
ACURATE Prime TF Delivery System XL is a slightly modified version of the ACURATE neo2 Transfemoral (TF) Delivery System.
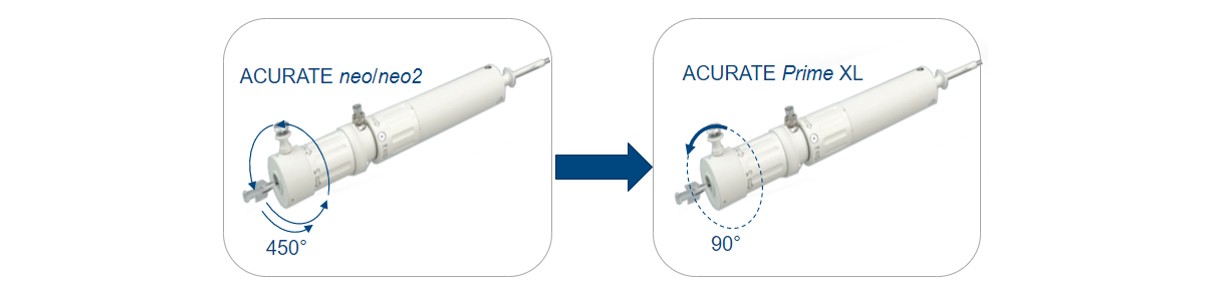
The system is compatible with the 14F iSLEEVE introducer and has a new distal release mechanism to allow quicker final valve release in one motion and improved valve detachment.