Hepatology: liver transplantation following Y-90 for HCC overview
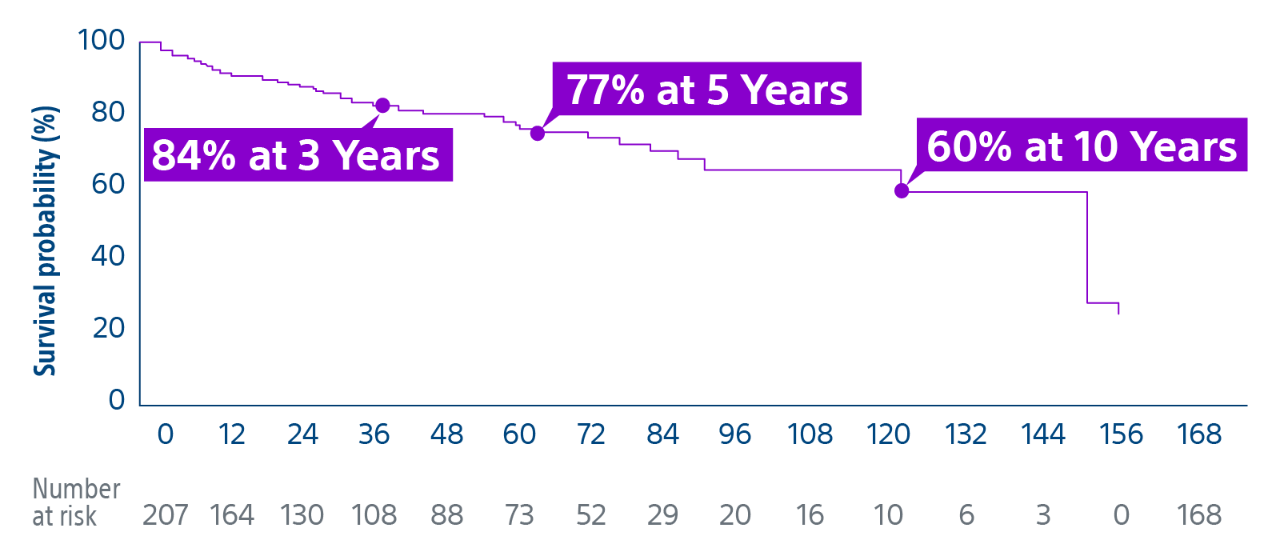
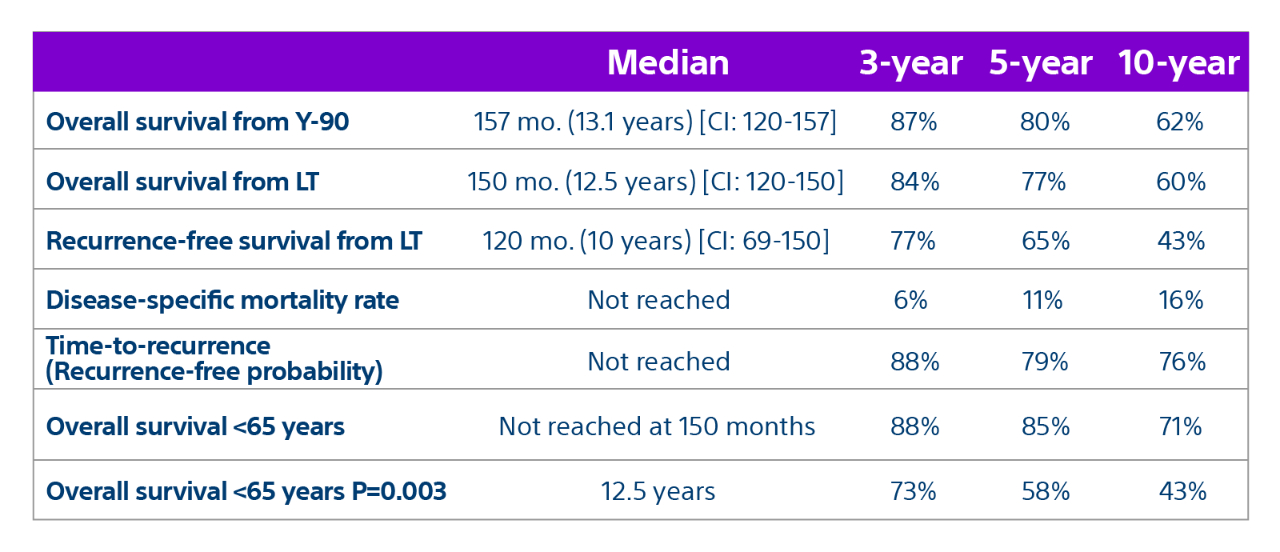
15-year, 207 patient experience highlights TheraSphere as an effective treatment for bridging or downstaging HCC to liver transplant therapy with a median overall survival* of 12.5 years.
*Post transplant
Liver transplantation following yttrium-90 radioembolization: 15-year experience in 207-patient cohort - Ahmed Gabr, Laura Kulik, Samdeep Mouli, Ahsun Riaz, Rehan Ali, Kush Desai, RonaldA Mora, Daniel Ganger, Haripriya Maddur, Steven Flamm, Justin Boike, Christopher Moore, Bartley Thornburg, Ali Alasadi, Talia Baker, Daniel Borja-Cacho, Nitin Katariya, Daniela P Ladner, Juan Carlos Caicedo, Robert J Lewandowski, Riad Salem
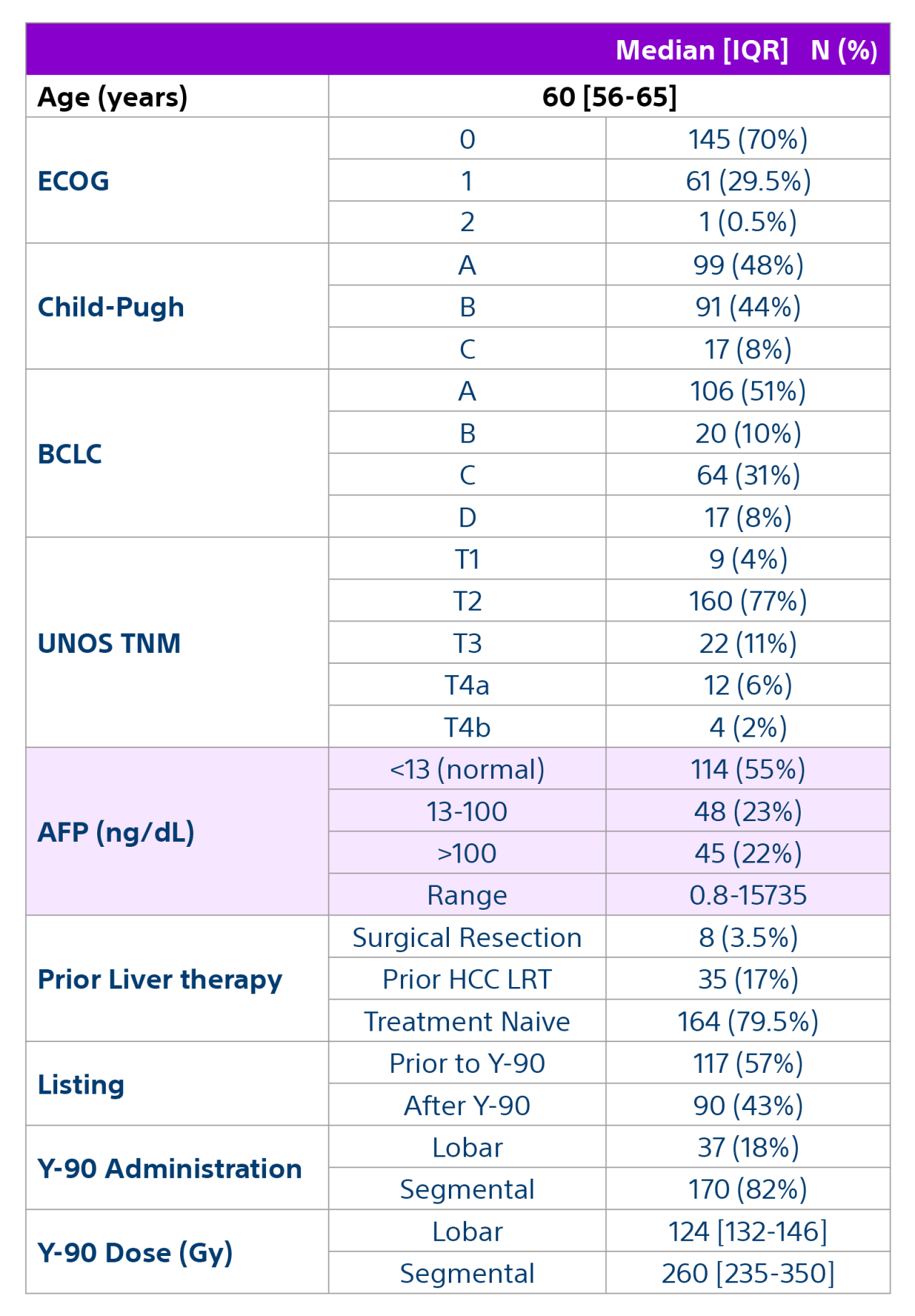
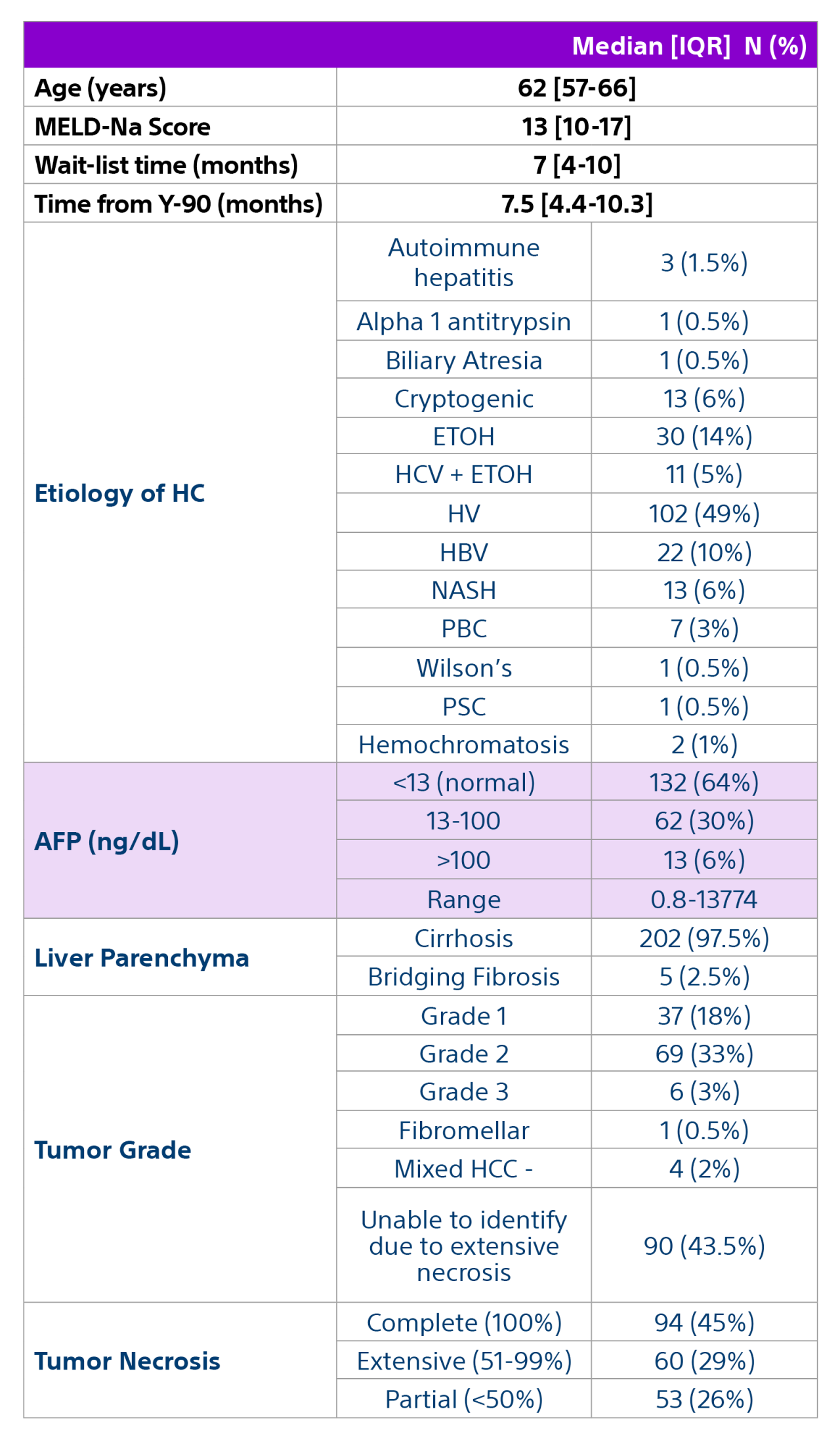
Methods: A multidisciplinary team comprised of hepatology, oncology, transplant surgery, and interventional radiology retrospectively reviewed data from 207 patients with unresectable HCC who underwent liver transplant after being treated with Y-90 as part of a bridging or downstaging care pathway.
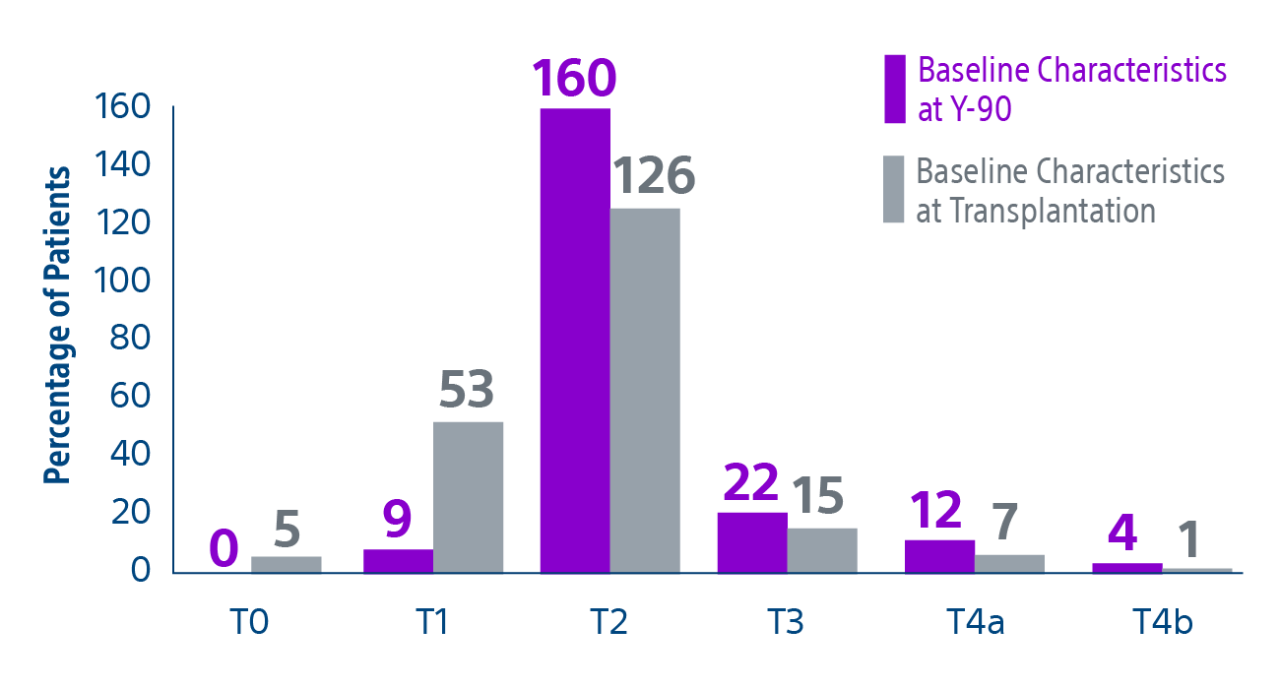
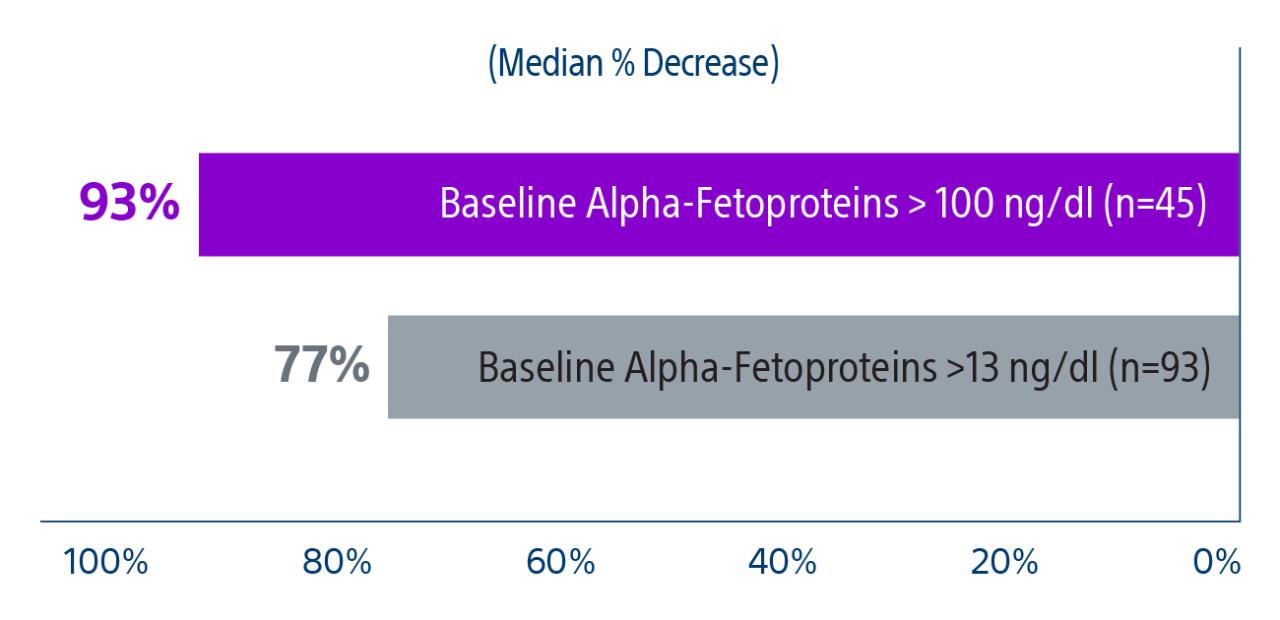
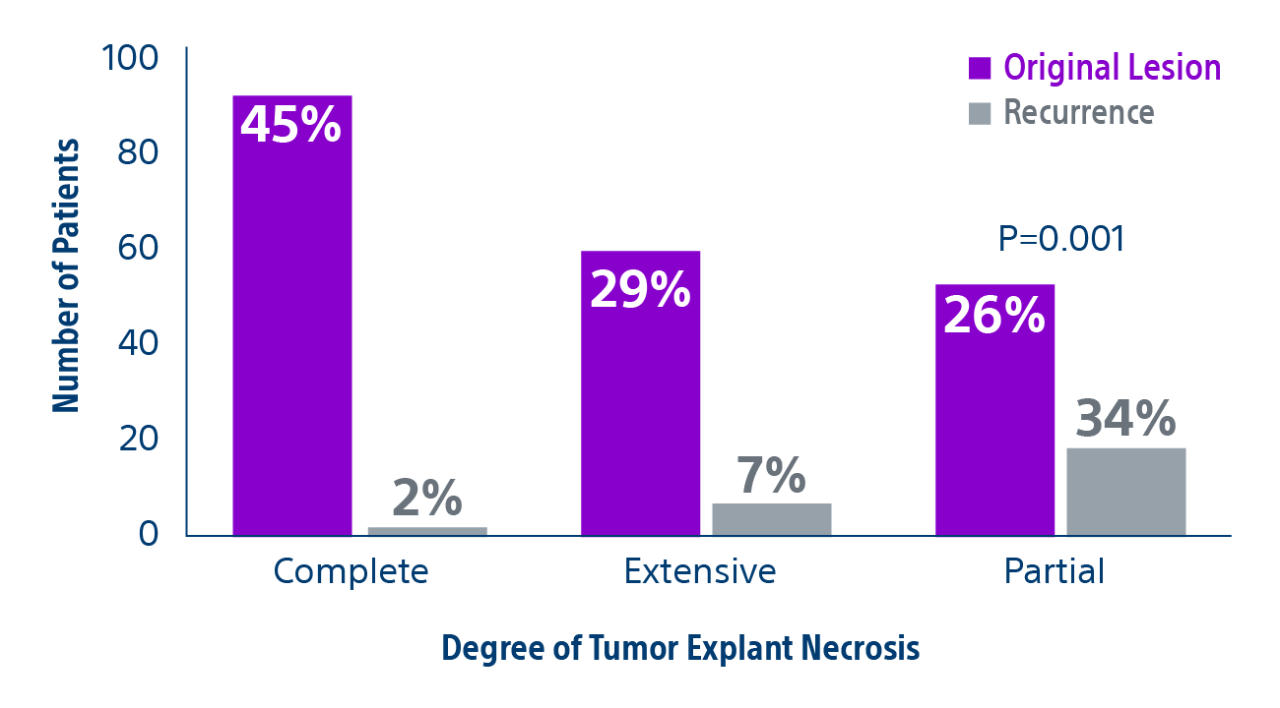
Results: Of the 207 patients included in these analyses, 38 (19%) patients were downstaged to within Milan transplant criteria and 169 (82%) bridged to transplant with TheraSphere either using lobar (18%, median dose of 124 Gy [IQR: 132-146]) or radiation segmentectomy (82%, median dose 260Gy [IQR: 235-350]) administration.