TCT 2021 - Structural Heart Valves
Symposia: Protecting Patients Today – Preserving Options for Life.
Moderated by Michael J. Reardon, MD and Samir Kapadia, MD, this session includes discussions on acute outcomes and lifetime patient management in TAVR procedures, including cerebral embolic protection, preserving coronary access, and clinical trials for the ACURATE neo2™ Valve..
Features discussants Suzanne Baron, MD; Helge Moellmann, MD; and Tanja Rudolph, MD.
Poster: Multicenter comparison of latest generation self-expanding versus balloon-expandable transcatheter aortic valves
At TCT, Dr. Won-Keun Kim presented results from a multicentery registry comparing the latest generation of supra-annular and intra-annular transcatheter heart valves, the ACURATE neo2 Transcatheter Aortic Valve Replacement System, and the SAPIEN 3 Ultra™ Valve.
The researchers concluded that, based on the registry results, short-term outcomes after TAVR using the ACURATE neo2 or SAPIEN 3 Ultra valves were overall comparable. Transprosthetic gradients were lower with the neo2 platform, which translated into a lower rate of device failure.
Study design: 722 patients at 4 centers were included and treated either with ACURATE neo2 (n=230) or Ultra (n=492) THV. Using 1-to-1 propensity score matching (PSM), 178 matched pairs were identified.
Results
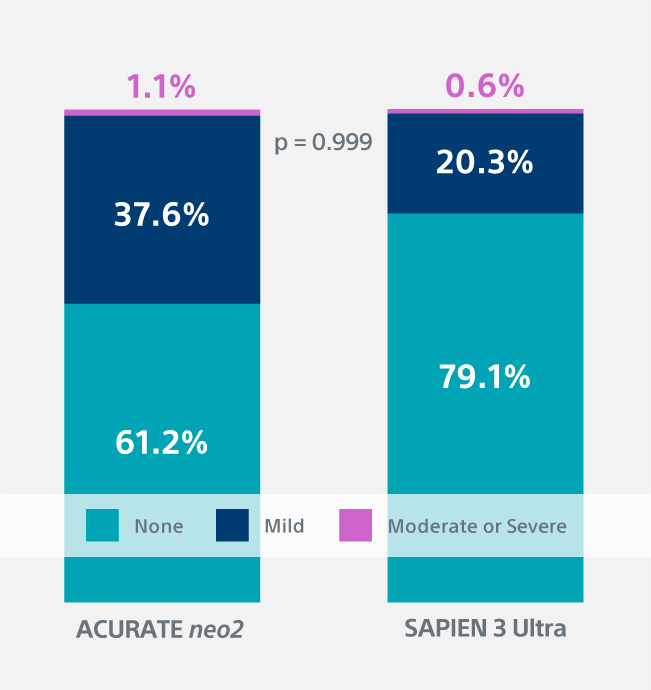
Paravalvular Leak1
1.1% moderate to severe PVL with the ACURATE neo2 Valve vs. 0.6% with the SAPIEN 3 Ultra Valve (p = 0.999)

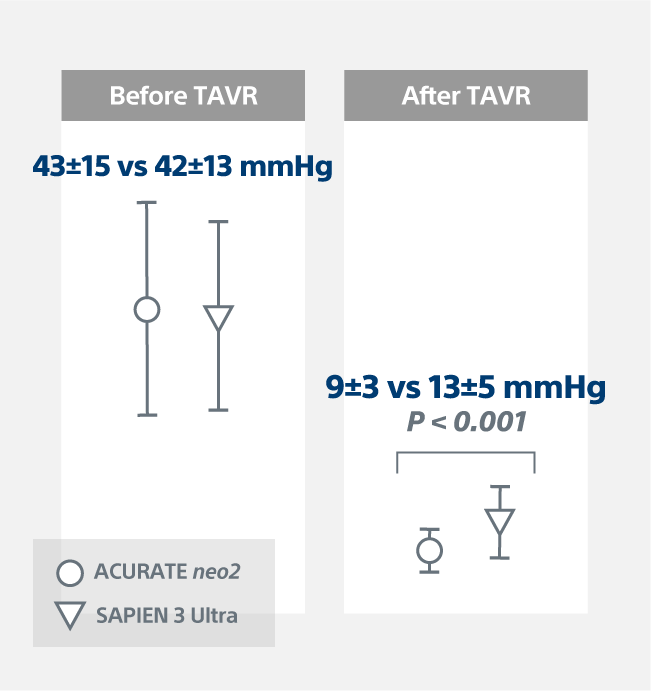
Hemodynamics1
0.6% elevated gradients with the ACURATE neo2 Valve vs. 7.3% with the SAPIEN 3 Ultra Valve (p = 0.003)
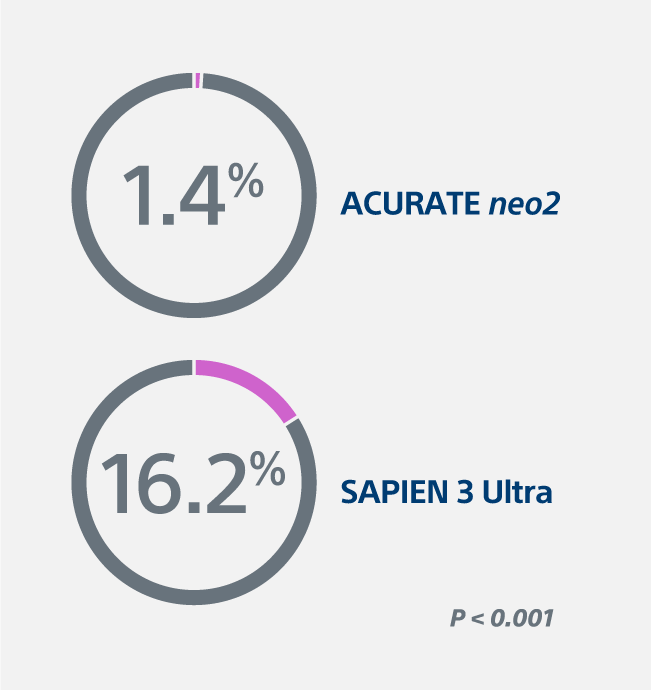
1.4% severe prosthesis-patient mismatch (PPM) with ACURATE neo2 Valve vs. 16.2% with the SAPIEN 3 Ultra Valve (P < 0.001)
Elevated gradients are ≥ 20mmHg
Mean Gradients
Severe PPM
1. Kim WK., et al. Multicenter comparison of latest generation of self-expanding versus balloon-expandable transcatheter aortic valves. TCT Congress. 2021.
Poster: Cost-Effectiveness of Cerebral Embolic Protection with the SENTINEL Device in Transcatheter Aortic Valve Replacement: a US Medicare Payer Perspective
Dr. Mohamad Alkhouli presented the abstract entitled Cost-Effectiveness of Cerebral Embolic Protection (CEP) with the SENTINEL Device in Transcatheter Aortic Valve Replacement: a US Medicare Payer Perspective at TCT 2021 on November 6. Results show at 5 years, CEP was associated with a lower mean cumulative cost to Medicare and greater quality-adjusted months alive than no-CEP. Dr. Alkhouli concluded that based on this analysis CEP in TAVR using the SENTINEL device is cost-effective from a Medicare payer perspective.
Background
- Previous observational studies have suggested an incremental increase in the cost of TAVR with routine CEP. However, cost-effectiveness analyses assessing the cost of CEP relative to its effects are not available.
Methods
- A decision-analytic model was constructed to simulate the outcomes and costs of TAVR with CEP (Sentinel; Boston Scientific Corporation; Marlborough, MA) vs. no-CEP.
- Probabilities of peri-procedural stroke were obtained from a patient-level analysis of 1,306 randomized or propensity-matched patients in which the incidence of stroke was 1.88% with CEP vs. 5.44% without CEP.
- Probabilities of death were obtained from an analysis of Medicare claims data (7/1/2016-6/30/2017) in which 490 patients who had post-TAVR stroke were propensity-matched to 2,429 patients without post-TAVR stroke.
- Utility inputs were obtained from the literature.
Results
- At 5 years, the modelled mean predicted quality-adjusted months alive was 29.4 for CEP and 28.7 for no-CEP.
- The cumulative cost to Medicare per patient was $147,711 for CEP and $148,711 for no-CEP (difference=$1,000).
- CEP was less costly and more effective when the mean results were considered.
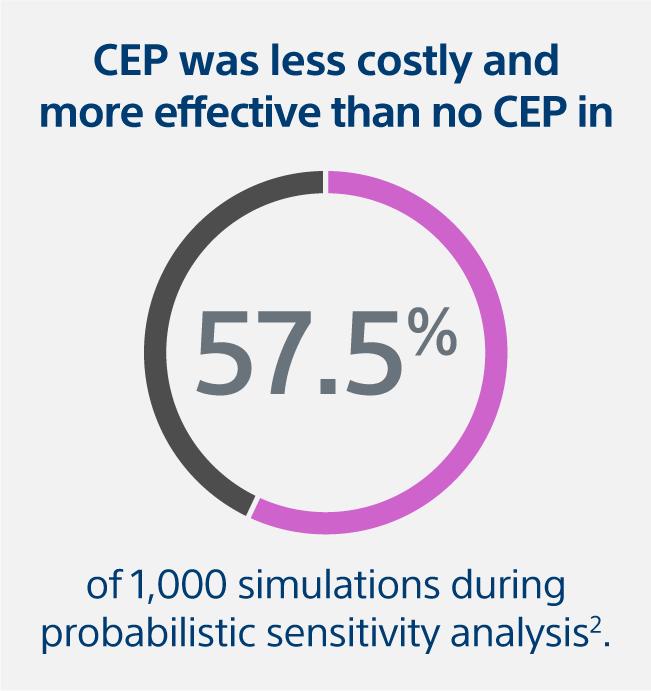
- In the probabilistic sensitivity analysis, CEP was less costly and more effective than no-CEP in 57.5% of the 1,000 simulations performed.
- At a willingness-to-pay threshold of $10,000 per quality-adjusted month alive, CEP with Sentinel was cost-effective (dominated or had a cost-effectiveness ratio of ≤$10,000 per quality-adjusted month alive) in 88.9%of simulations.
- These results remained similar in sensitivity analyses with different incidences of post-TAVR strokes.
Conclusion
- CEP in TAVR using the SENTINEL device is projected to be economically cost-effective from a Medicare payer perspective.
2. Alkhouli M., et al. Cost-Effectiveness of Cerebral Embolic Protection with the SENTINEL Device in Transcatheter Aortic Valve Replacement: a US Medicare Payer Perspective. TCT Congress. 2021.
CAUTION: In Europe, ACURATE neo and neo2 Aortic Valve Systems are CE-marked. In the USA, ACURATE neo2 is an investigational device and restricted under federal law to investigational use only. Not available for sale. Products shown for informational purposes only – not meant as promotion or offer for sale.