Boston Scientific accounts are for healthcare professionals only.

Varithena™ (polidocanol injectable foam) 1%
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical Data
- Technical Specifications
- Ordering information
- Training
- Resources
One thing can be a meaningful addition for your patients and your practice.
Varithena is the only FDA-approved foam indicated for treating chronic venous insufficiency (CVI), using a non-thermal, non-tumescent approach for the great saphenous vein (GSV) system and its associated veins. Its patented Microfoam UDSS™ technology produces a cohesive, low-nitrogen microfoam (O2:CO2 ratio of 65:35 with <0.8% nitrogen) and takes approximately 10 minutes to administer. The microfoam fills the vein lumen for circumferential contact, displacing blood and efficiently destroying the endothelial lining.
Varithena mechanism of action explained
- Incompetent tributaries can be treated through either one injection site, or when warranted, multiple injection sites.
- A column of microfoam advances to fill the vein.
- Varithena displaces blood, effectively filling the lumen for circumferential contact.1
- Varithena achieves endothelial destruction with very low polidocanol concentration.
- The vein contracts, narrowing the lumen until vein has almost no volume.
- Residual, low-nitrogen bubbles are highly absorbable in blood and are swept away and absorbed in venous circulation.
Add versatility to your practice.
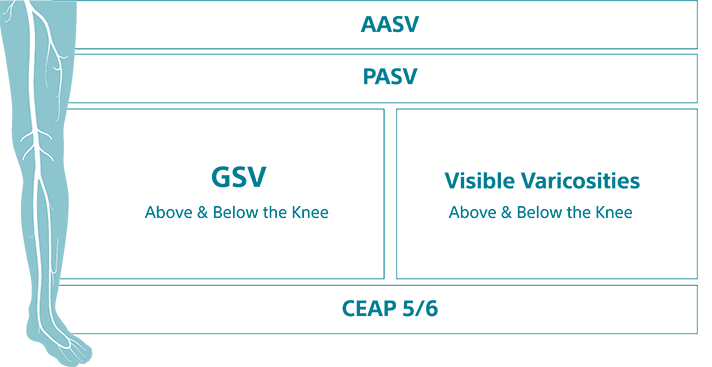
GSV above and below the knee, tortuosity, recanalization, large and small veins C2-C6. Treat them all with Varithena.
- Recurrent veins
- Above and below the knee
- Recanalization
- Wide range of diameters up to 25.9 mm
- Tortuosity
Interested in adding Varithena to your practice?
Learn more about becoming Varithena certified.
References:
1. Eckmann DM. Polidocanol for endovenous microfoam sclerosant therapy. Expert Opin Investig Drugs. 2009 Dec;18(12):1919-27. doi: 10.1517/13543780903376163.
Network Meta-Analysis
The ongoing network meta-analysis (NMA) evaluates the evidence for Varithena (PEM) compared to endovenous thermal ablation (ETA) in treating chronic venous insufficiency (CVI). By distinguishing Varithena, the only FDA-approved, commercially available non-compounded polidocanol 1% endovenous microfoam ablation, from other foam sclerotherapy options, this NMA provides more precise and generalizable evidence on the relative effectiveness and safety of these treatments.
Varithena’s record of safety and efficacy isn’t limited to clinical studies.
These key findings are from a collection of real-world studies and peer-reviewed journals.
A low-nitrogen solution that leads to increased safety and performance.
Easy and efficient to use for you and your staff to use.
Varithena’s low-nitrogen microfoam comes pre-formulated and delivers a reliably cohesive performance. The small, residual bubbles are rapidly absorbed for consistent results with reduced complications.
- O2:CO2 (65:35) gas mixture with <0.8% nitrogen
- Reliably small bubbles (median diameter <100 µm; all ≤500 µm)
- 7:1 gas: liquid ratio enhances blood displacement to allow for longer dwell time in the vessel
text test
Varithena microfoam

Physician-compounded microfoam
See how the microfoam is generated.
This video explains how the canister device is activated to dispense cohesive, low-nitrogen microfoam.
Ordering information
Varithena can be ordered directly through Boston Scientific.
First time ordering Varithena?
If this is your first time ordering Varithena, please note that you must become Varithena certified prior to ordering. To learn more information about becoming certified, please click below!
Returning Varithena customer?
Complete an order form by clicking below!
Become Varithena certified
Join the 3,000+ certified physicians improving patients' lives with Varithena. We're here to help you, and becoming certified is easy.
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Insurance coverage for Varithena
Varithena is included in a majority of regional and national payor's polices and local coverage decisions.1
References
1. Data on file. Provensis Ltd, 2013.