Boston Scientific accounts are for healthcare professionals only.

Infinion™ Pro Percutaneous Leads
Reimbursement
Configure or select a product to continue to order
- Overview
- Technical specifications
- Ordering information
- Training
- Resources
Designed to evolve with your patients’ treatment needs
The Infinion Pro Percutaneous Leads are designed to deliver confident coverage, proven relief, and enhanced performance and durability.

Why choose Infinion Pro Percutaneous Leads
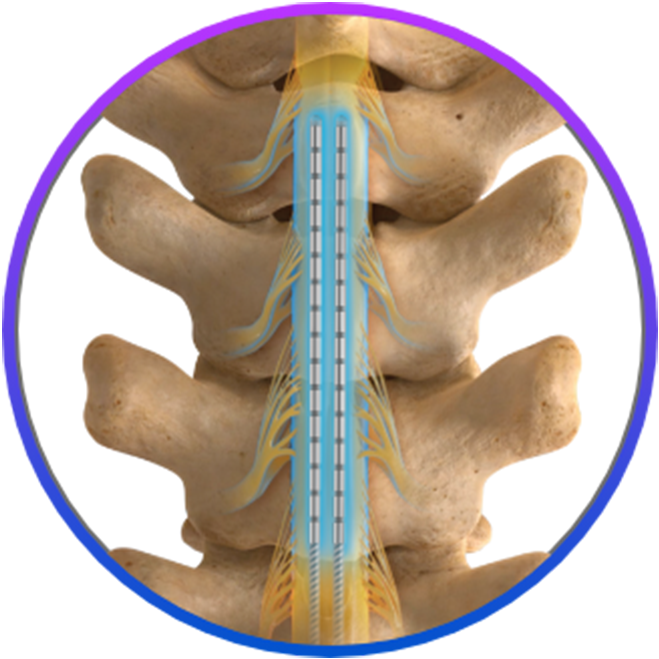
Confident coverage
- Spans 3 vertebral levels and provides seamless coverage of multiple pain targets with tight contact spacing
- Allows physicians to easily find and keep the optimal target for patients, including diabetic peripheral neuropathy patients* and patients with multiple areas of pain
- Designed to mitigate loss of therapy in the event of lead migration or evolution of pain pattern
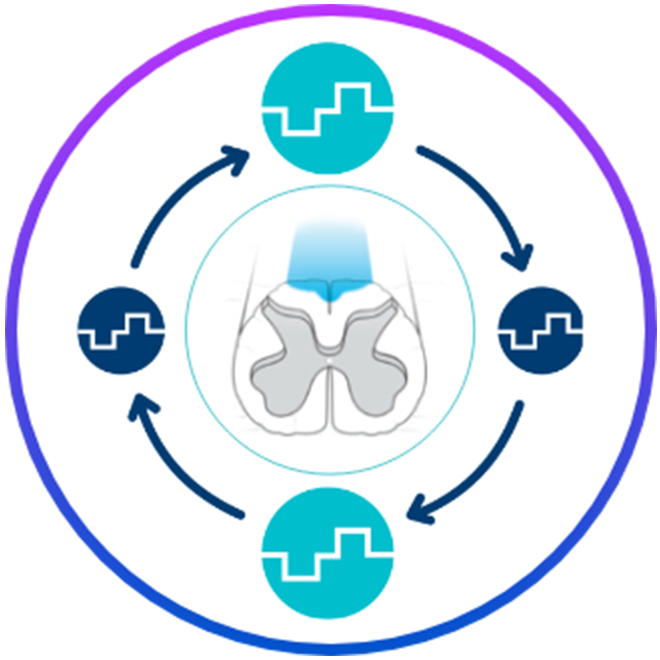
Proven relief
- Access the latest therapies, including FAST™ AutoDose for sustained profound, paresthesia-free relief in as fast as approximately 2 minutes1-3
- MRI conditionality to maintain access to pain relief**
Enhanced performance and durability
- Backed by an industry leading 3-year warranty, the Infinion Pro Leads are over 10x more durable than the market leading tight-spaced percutaneous lead bodies4
- New coiled lead body designed for optimized steerability and handling
How it works
View an overview of the surgical procedure with the Infinion Pro Lead and innovative Entrada™ Needle.
Subscribe for updates on Spinal Cord Stimulation
Receive timely access to major announcements, opportunities to connect with your peers through educational events, and useful tools for you to help more patients.
Infinion™ Pro Percutaneous Leads
Technical specifications
| FEATURE | SPECIFICATIONS |
|---|---|
| Lead Lengths | 50cm, 70cm |
| Lead Diameter | 1.3mm |
| Number of Contacts | 16 contacts |
| Span | 67mm |
| Content Length | 3mm |
| Contact Spacing | 1mm |
Ordering information
| MODEL NUMBER | UPN | DESCRIPTION |
|---|---|---|
| SC-2318-50 | M365SC2318500 | Infinion Pro 50cm 16 Contact Lead Kit |
| SC-2318-70 | M365SC2318700 | Infinion Pro 70cm 16 Contact Lead Kit |
| SC-4220-45 | M365SC4220450 | Entrada Needle 11cm (4.5in) |
| SC-4220-60 | M365SC4220600 | Entrada Needle 15cm (6.0in) |
| SC-4220-S45 | M365SC4220S450 | Entrada Spare Sheath 11cm (4.5in) |
| SC-4220-S60 | M365SC4220S600 | Entrada Spare Sheath 15cm (6.0in) |
| SC-4318 | M365SC43180 | Clik™ X Anchor |
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Resources
To explore our comprehensive portfolio of therapies for chronic pain, visit BostonScientific.com/Pain.
For reimbursement support, access coding and payment guides.
*Boston Scientific Spinal Cord Stimulator Systems are indicated for use in patients with painful diabetic peripheral neuropathy (DPN) of the lower extremities only with paresthesia-based therapies. FAST™ Therapy and other sub-perception modalities, including Combination Therapy, have not been approved by the FDA for DPN. Do not promote the use of sub-perception modalities with BSC devices in DPN patients. If a prescribing physician decides to use FAST, other sub-perception therapies or Combination Therapy, BSC may provide technical support on the use of the product as directed by the physician.
**MRI Conditional under specified conditions
The WaveWriter Alpha™ SCS System provides safe access to full-body MRI scans when used with specific components and the patient is exposed to the MRI environment under the defined conditions in the ImageReady™ MRI Full Body Guidelines for WaveWriter Alpha and WaveWriter Alpha Prime Spinal Cord Stimulator System.
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
Sub-perception stimulation has been demonstrated to be safe and effective in patients who have been treated successfully with conventional, paresthesia-inducing stimulation for at least six months. Full stimulation parameter ranges and options for both paresthesia based and sub-perception therapy are available for clinician’s use throughout the patient’s experience and treatment with SCS.
References:
- Gage E., et al. Rapid Onset of Analgesia during Trial Period Utilizing Fast-Acting Sub-Perception Therapy SCS [Abstract] NANS 2023 Annual Meeting, Jan 12-15, Las Vegas, NV.
- Anitescu M., et al. Prospective, Multicenter Outcomes Utilizing an SCS-System Designed to Engage Surround Inhibition Using Fast-Acting Sub-Perception Therapy [Abstract]. Sixteenth World Congress of the International Neuromodulation Society, May 11-16th, 2024, Vancouver, BC Canada.
- Ferro R., et al. Personalized Spinal Cord Stimulation Therapy Via Automated Neural Dosing (FAST AutoDose) [Abstract]. Sixteenth World Congress of the International Neuromodulation Society, May 11-16th, 2024, Vancouver, BC Canada.
- Data on file.
