Boston Scientific accounts are for healthcare professionals only.

AGENT™ Drug-Coated Balloon
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Ordering information
- Training
- Reimbursement
- Resources
FDA approved: An AGENT of change for ISR treatment
AGENT Drug-Coated Balloon expands treatment options for physicians and their patients by delivering a targeted anti-proliferative drug dose, without introducing an extra layer of metal.
How it works
Why choose AGENT Drug-Coated Balloon?
ISR treatment option expansion
~10% of PCIs are complicated by in-stent restenosis (ISR)1. Currently, 82% of patients with ISR receive another stent.2 Yet repeat intervention is associated with a higher risk of target lesion revascularization.3
For patients with ISR, placing additional stents increases risks including thrombosis⁴ and future ISR.⁵ ⁶ Coronary drug-coated balloons (DCBs) provide a proven alternative and have been used to treat more than 1 million patients worldwide.⁷
Comparing DCB to DES when treating ISR
DCBs present an advantage when compared to bare-metal stents and drug-eluting stents in that they do not introduce an additional metal layer that may cause potential complications (including fracture, malposition, and thrombosis).⁵
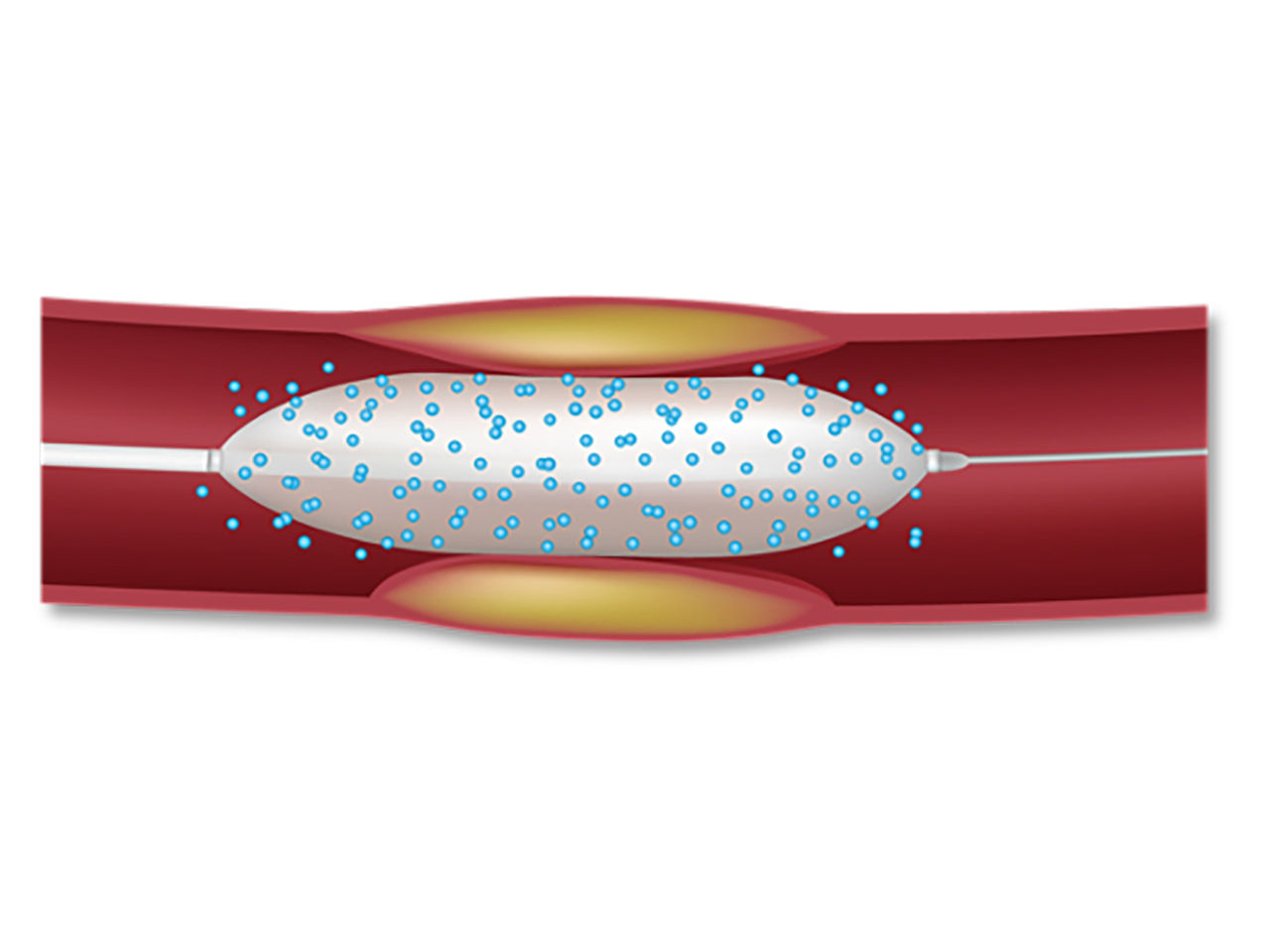
Drug-Coated Balloon (DCB)
| Excipient maintains coating integrity, but is less protective than DES Polymer |
| Single high dose transfer of at least 30 seconds |
| No metal left behind |

Drug-Eluting Stent (DES)
| Polymer protects drug during tracking |
| Polymer controls sustained drug release for 90+ days* |
| Permanent presence of stent |
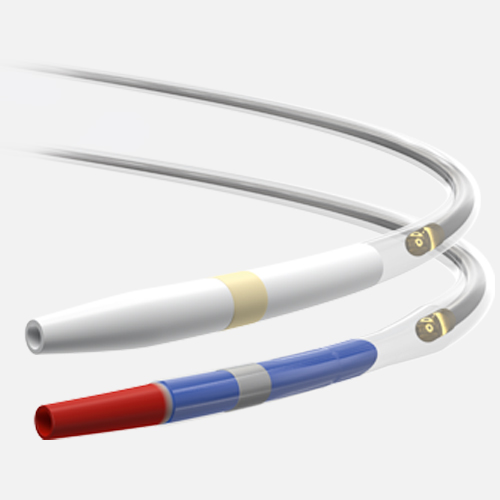
The right drug. The right delivery method.
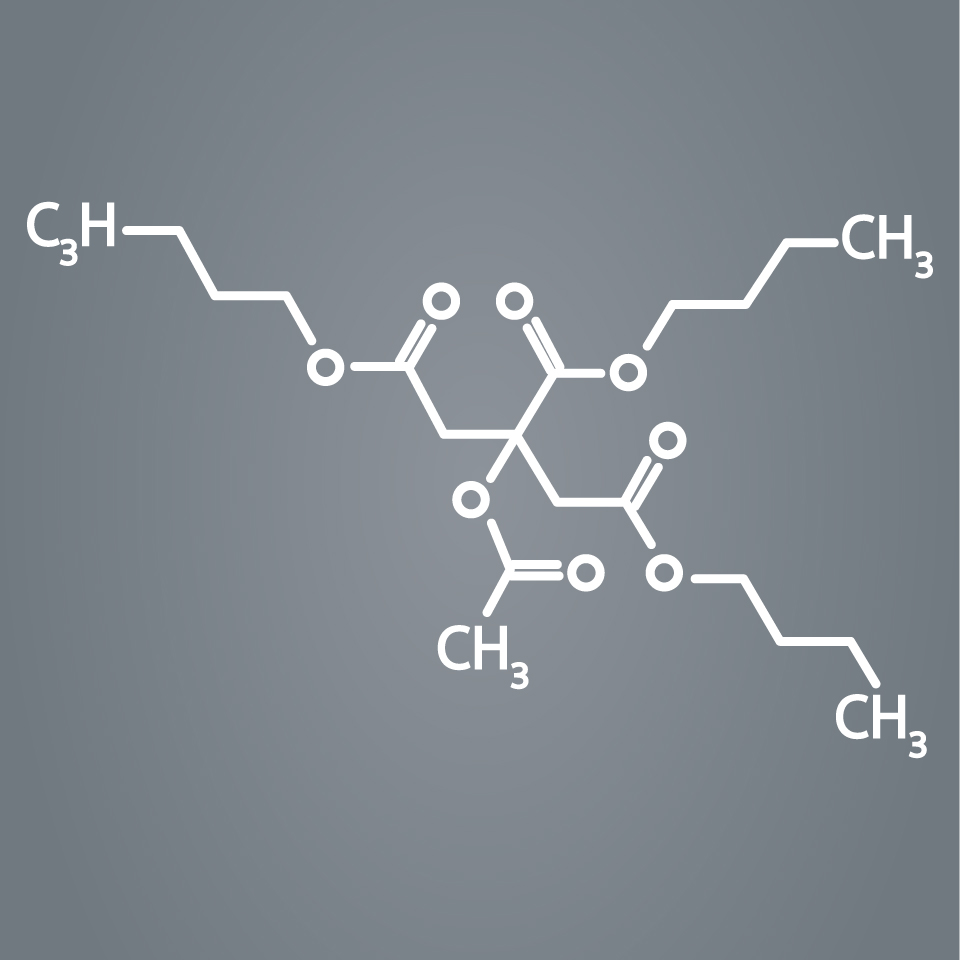
AGENT is designed with a novel excipient, sharp-edge structure, and uniform crystalline formulation. Its proprietary TransPax™ coating technology optimizes the three phases of drug delivery: transfer, absorption, and retention.
Paclitaxel, the drug used in AGENT and the majority of DCBs worldwide, is better suited for a Coronary DCB Application than Sirolimus:
TransPax™ Coating Technology
With the right balance of hydrophilic and hydrophobic characteristics, our proprietary TransPax Coating Technology is designed to maximize drug transfer to the target vessel wall and delivers the right amount of treatment exactly where it’s needed.
TransPax coating is a combination of:
- Low dose paclitaxel at 2µ/mm2
- Novel excipient Acetyl Tributyl Citrate (ATBC)
- Proprietary manufacturing process

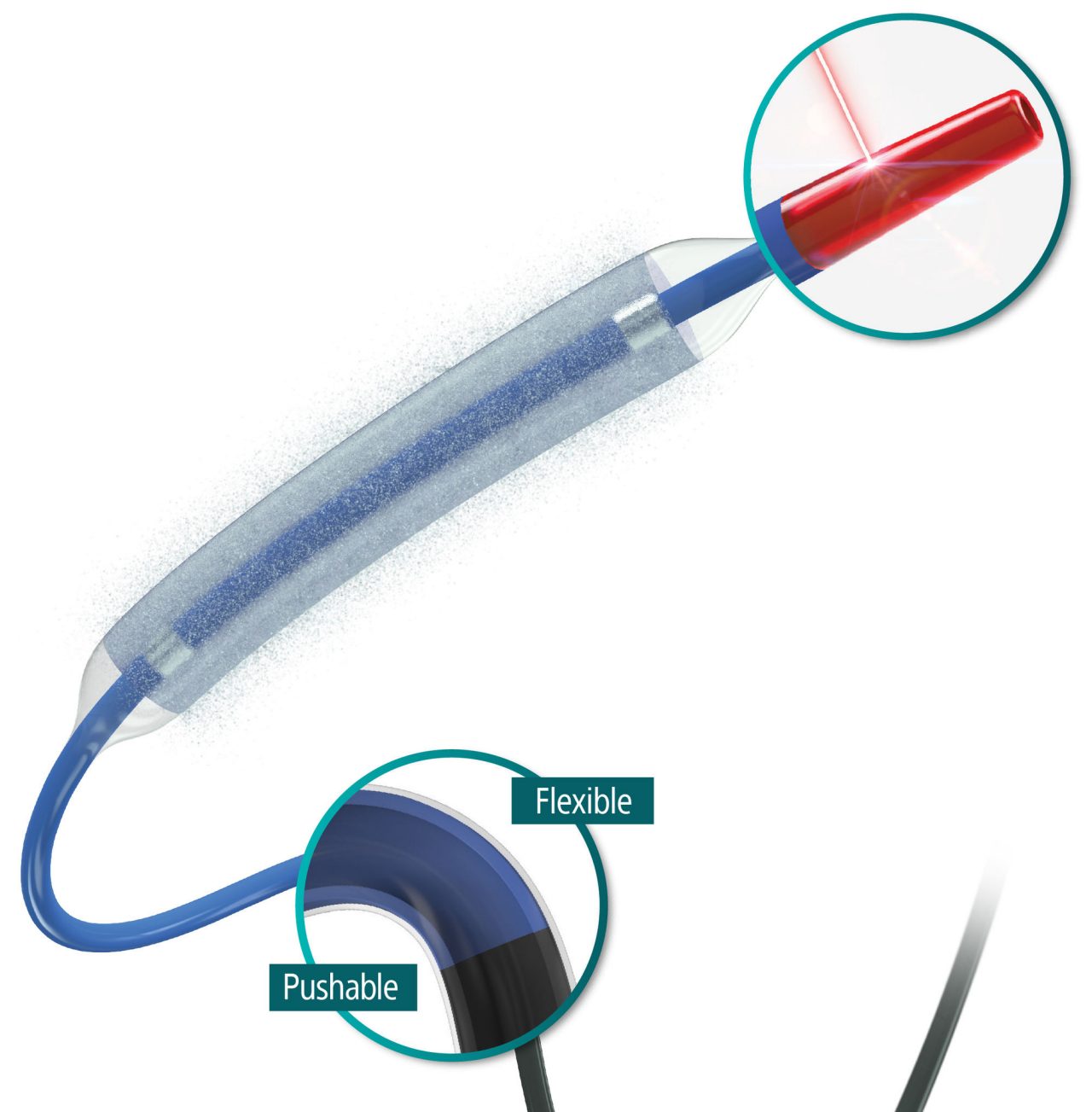
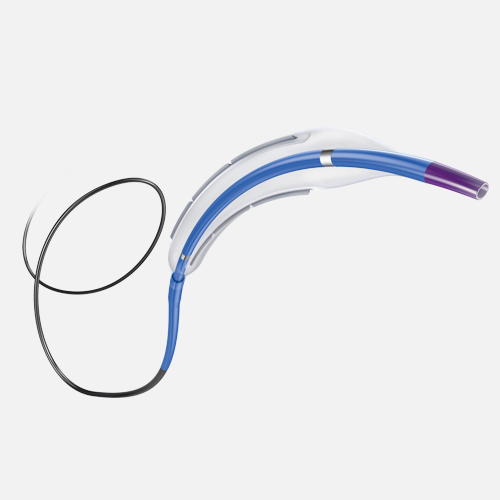
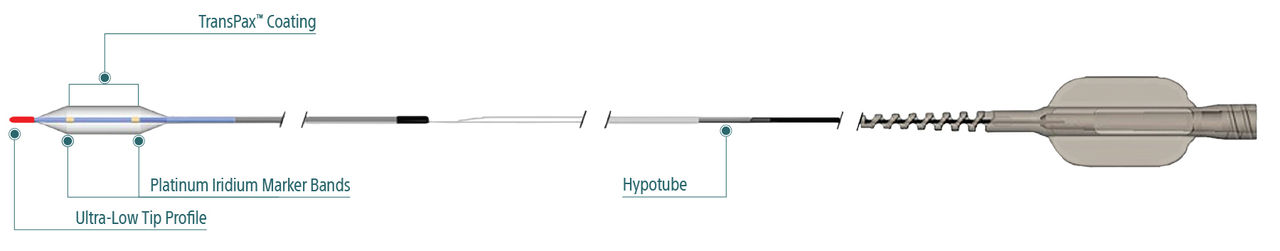
Exceptional deliverability
AGENT reduces time to lesion, which allows for maximized drug tissue concentration and minimized downstream particulates, reducing the risk of embolization. Features include:
Laser Bonded Tip
- Improves crossability and reduces tip catch.
Bi-segment Inner Shaft
- Improves trackability in tortuous distal anatomy
Z-glide™
- Improves tracking with proprietary lubricious coating
TransPax™
- Efficient coating technology
Discover the Modern PCI approach
See the vessel
Assess plaque type and severity using the latest imaging and physiology technology. Eliminate guesswork and make clearer treatment decisions.
Prep the vessel
Successful outcomes start with proper lesion assessment and vessel preparation. Achieve optimal lumen gain with our cutting balloon and rotational atherectomy tools.
Treat the vessel
With our innovative stent portfolio and coronary drug-coated balloon technology, you have access to market-leading therapies that will ensure you are able to improve long-term patient outcomes.
Subscribe for updates on AGENT DCB
Receive timely updates on significant announcements, exclusive opportunities to engage with peers through educational events, and access valuable tools to enhance your ability to assist more patients.
Clinical highlights
Recent clinical data continues to reinforce AGENT™ Drug-Coated Balloon (DCB) as a proven solution for treating coronary artery disease. AGENT DCB has been used in more than 275,000 patients worldwide and studied in more than 16,000 patients across complex in-stent restenosis (ISR) and de novo populations.15
2024
AGENT IDE Trial
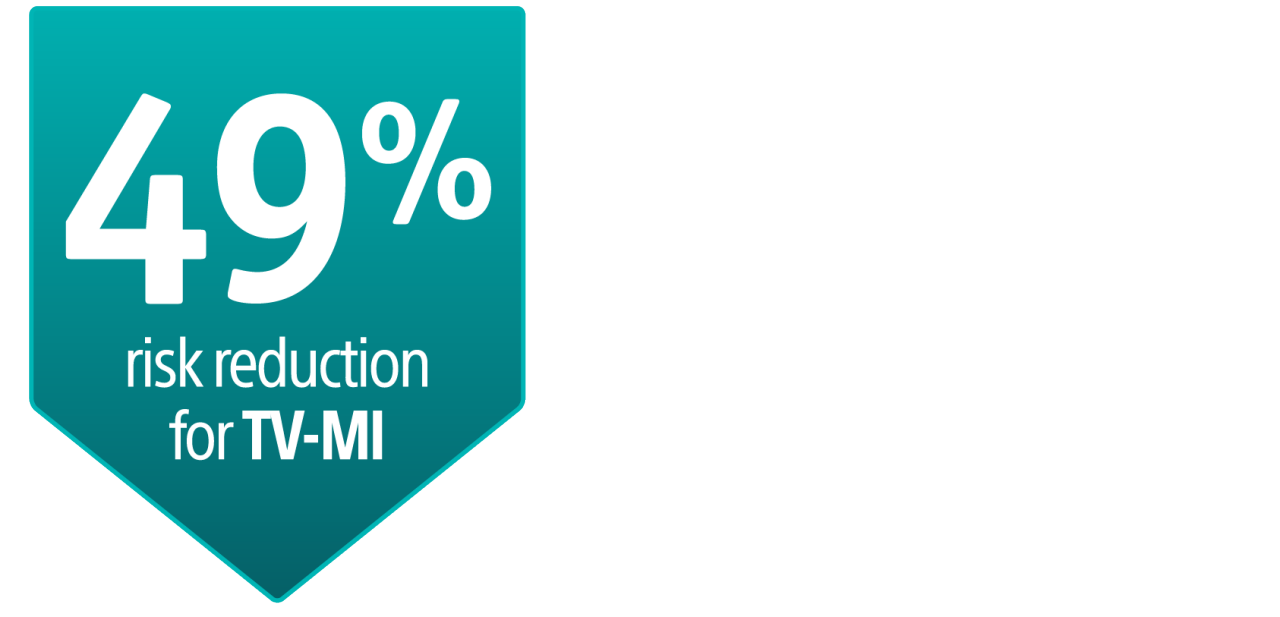
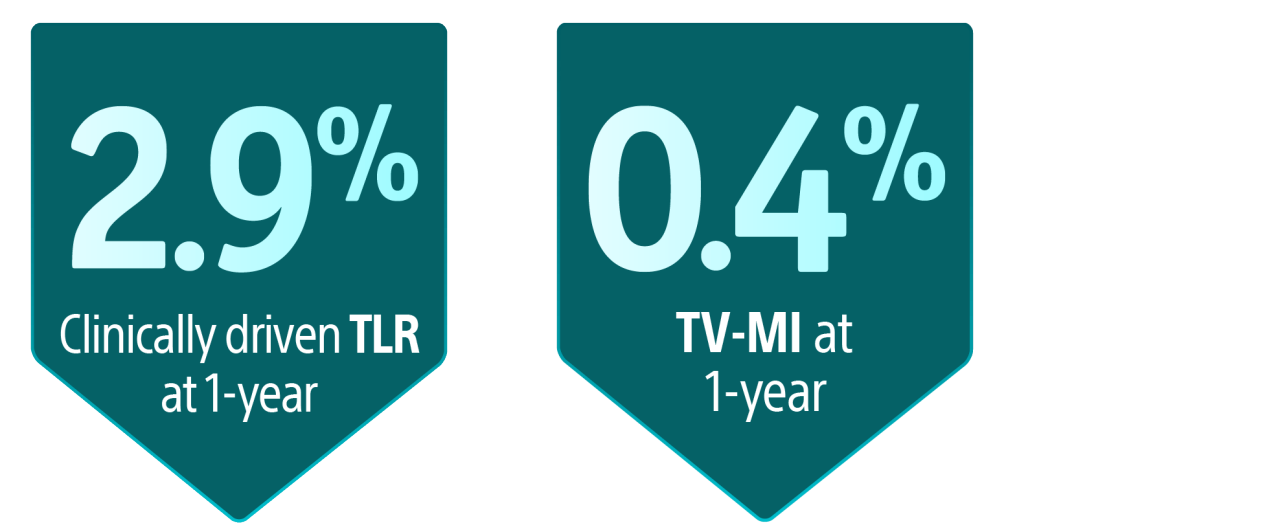
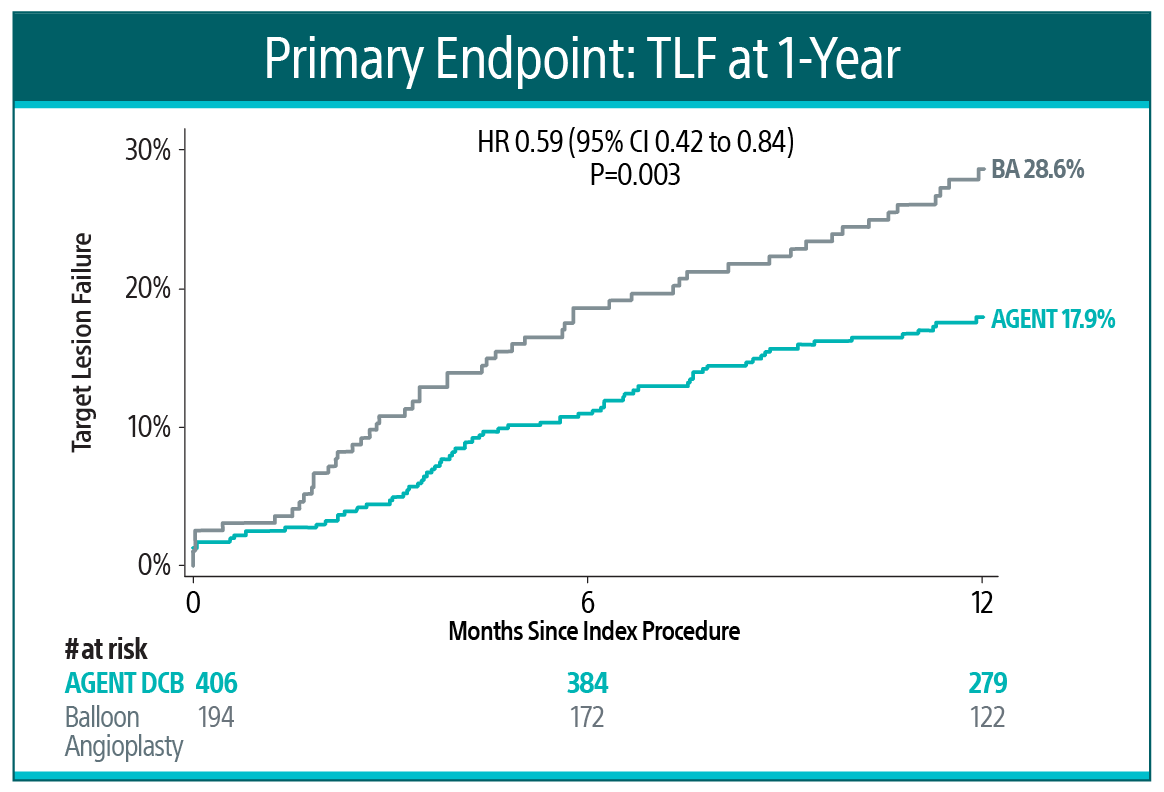
AGENT IDE is a prospective, multicenter, randomized controlled trial in the United States to evaluate the safety and effectiveness of the AGENT DCB compared to balloon angioplasty in patients with in-stent restenosis (ISR).16 At one year, AGENT DCB demonstrated statistically lower event rates16:
Primary Endpoint17
AGENT DCB showed statistically superior outcomes compared to balloon angioplasty for TLF at 1-year (17.9% versus 28.6% P= 0.003).
TLF relative risk reduction from using AGENT DCB was approximately 41%.

2025
Post-Approval Study
Data from over 12,337* real-world AGENT DCB patients in the U.S. demonstrates the rapid uptake of the therapy and excellent in-hospital safety outcomes comparable to Drug-Eluting Stent (DES) patients.18
In-hospital safety outcomes for ISR PCIs**
All-cause mortality

Myocardial infarction (MI)
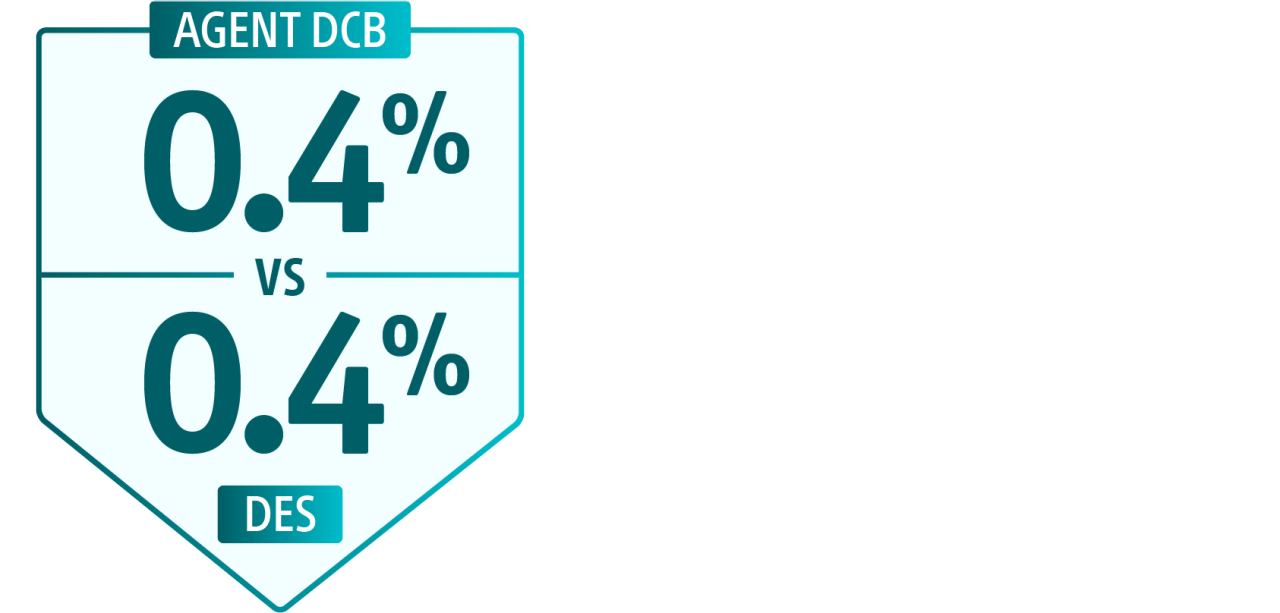
Bleeding

Heart failure

Ischemic stroke

This study showed approximately 17.5% of patients undergoing ISR PCI are now treated with a DCB. It also found DCB is more often used with intravascular imaging and adjunctive prep devices.18
*27.2% of AGENT ISR use (3,459/12,729 procedures) through June 2025 was for an off-label indication. These findings are presented for scientific discussion only. Please refer tothe Instructions for Use (IFU) or product labeling for approved indications and usage.
**AGENT DCB n=9,269 and DES n=65,890.
2025
ALLIANCE Registry*
The ALLIANCE Registry is a prospective, non-randomized, multicenter, all-corners de novo registry of over 1,800 patients including over 1,300 patients treated with AGENT™ DCB.19
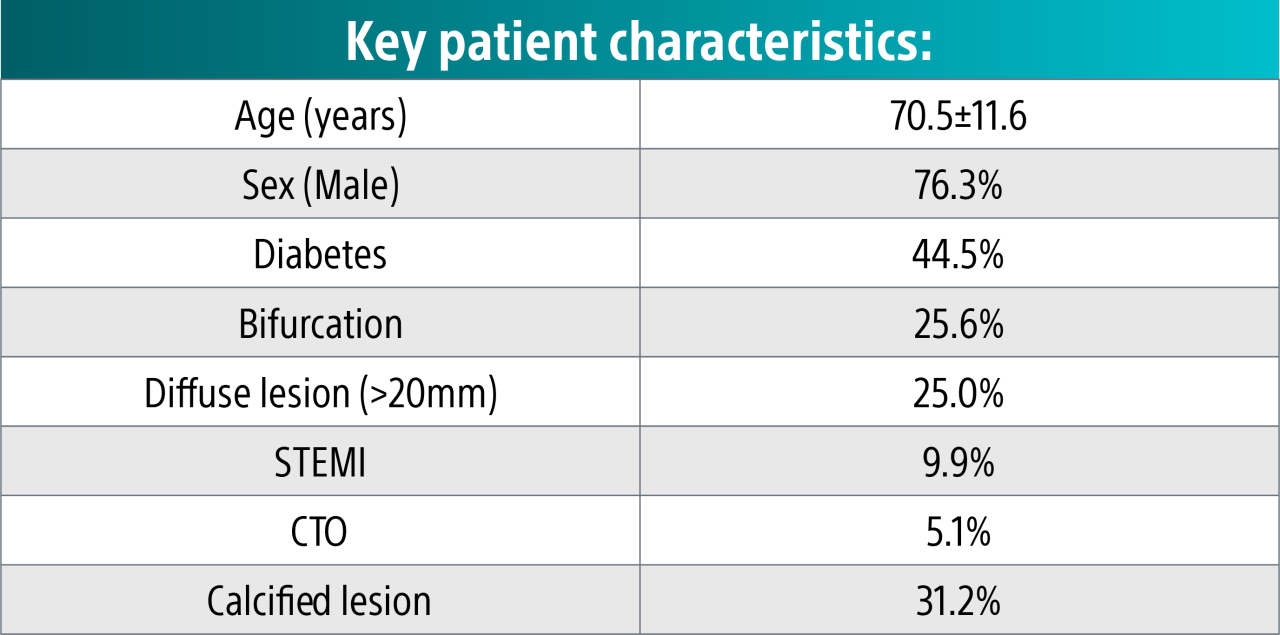
Complex patient population19
These clinical pages were observed despite a complex population and high rates of calcified lesions.
*Data shown may include results from studies using the product outside its approved labeling. These findings are presented for scientific discussion only. Please refer to the Instructions for Use (IFU) or product labeling for approved indications and usage.
Expanding Clinical Evidence Beyond ISR
AGENT DCB offers an alternative treatment option for patients with ISR. However, the majority of PCI procedures performed are in de novo lesions. Today, patients with de novo coronary lesions will often receive balloon angioplasty or a drug-eluting stent as the standard of care in the U.S.
Boston Scientific is dedicated to transforming lives through innovative medical solutions that improve the health of patients around the world. 275,000+ patients have been treated with AGENT DCB globally7, and over 16,000 patients studied with AGENT DCB.15 Patients are at the center of everything we do as we continue to research and advance science for life. That’s why Boston Scientific sponsored the AGENT DCB STANCE Trial.
This trial is designed to assess the safety and effectiveness of the AGENT DCB compared to the standard of care – percutaneous coronary intervention (PCI) treatment with drug-eluting stents (DES) and/or balloon angioplasty – in patients with de novo coronary lesions.
Patients enrolling in the trial must have a de novo target lesion located in a native coronary artery. The study is examining de novo lesions in small vessels, bifurcations, and long lesions. The primary endpoint is Target Lesion Failure (TLF) at 12 months.
Visit clinicaltrials.gov for more information on trial sites and expected completion.
Featured clinical publications
The American College of Cardiology (ACC) Interventional Council
In February 2023, The American College of Cardiology (ACC) Interventional Council released the recommendation that all cardiac cath labs should have imaging capabilities. The review proposes PCI best practices and advocates for broader use of IVI technologies.
RENOVATE-COMPLEX-PCI study
The recent RENOVATE-COMPLEX-PCI study provides evidence in favor of using intravascular imaging-guided PCI to address complex coronary artery lesions. At the 3-year follow-up, intravascular imaging-guided PCI demonstrated a reduced risk of target vessel failure when compared to angiography-guided PCI.¹
Modern PCI clinical data
Discover more clinical data for intravacular ultrasound (IVUS), FFR, and vessel preparation.
Technical specifications
Compliance Chart
| Ballon Length (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Pressure atm (kPa) | 2.00 | 2.25 | 2.50 | 2.75 | 3.00 | 3.50 | 4.00 |
| 3.0 (304) | 1.86 | 2.06 | 2.28 | 2.53 | 2.76 | 3.19 | 3.66 |
| 4.0 (405) | 1.93 | 2.14 | 2.37 | 2.61 | 2.85 | 3.30 | 3.80 |
| 5.0 (507) | 1.99 | 2.20 | 2.44 | 2.68 | 2.93 | 3.39 | 3.88 |
| 6.0 (608) | 2.03* | 2.26* | 2.50* | 2.75* | 3.00* | 3.46* | 3.96* |
| 7.0 (709) | 2.07 | 2.31 | 2.55 | 2.81 | 3.06 | 3.52 | 4.04 |
| 8.0 (811) | 2.10 | 2.34 | 2.59 | 2.85 | 3.11 | 3.57 | 4.09 |
| 9.0 (912) | 2.13 | 2.38 | 2.62 | 2.88 | 3.15 | 3.61 | 4.14 |
| 10.0 (1013) | 2.15 | 2.40 | 2.65 | 2.91 | 3.18 | 3.64 | 4.18 |
| 11.0 (1115) | 2.18 | 2.42 | 2.67 | 2.94 | 3.21 | 3.68 | 4.22 |
| 12.0 (1216) | 2.19 | 2.44 | 2.69 | 2.96 | 3.23 | 3.72** | 4.25** |
| 13.0 (1317) | 2.21 | 2.46 | 2.72 | 2.99 | 3.26 | ||
| 14.0 (1419) | 2.23** | 2.48** | 2.74** | 3.02** | 3.28** | ||
* Nominal Pressure
** Rated Burst Pressure. Do Not Exceed.
Modern PCI
The most complete Modern PCI portfolio in the industry
Ordering information
| Balloon Diameter (mm) | Balloon Length (mm) | |||
|---|---|---|---|---|
| E (mm) | 12 | 15 | 20 | 30 |
| 2.00 | H7493960812200 | H7493960815200 | H7493960820200 | H7493960830200 |
| 2.25 | H7493960812220 | H7493960815220 | H7493960820220 | H7493960830220 |
| 2.50 | H7493960812250 | H7493960815250 | H7493960820250 | H7493960830250 |
| 2.75 | H7493960812270 | H7493960815270 | H7493960820270 | H7493960830270 |
| 3.00 | H7493960812300 | H7493960815300 | H7493960820300 | H7493960830300 |
| 3.50 | H7493960812350 | H7493960815350 | H7493960820350 | H7493960830350 |
| 4.00 | H7493960812400 | H7493960815400 | H7493960820400 | H7493960830400 |
Modern PCI
The most complete Modern PCI portfolio in the industry
Education for AGENT DCB

Learn from top operators about the use of the AGENT Drug-Coated Balloon (DCB) in Modern PCI. This video series, with cardiology experts, will take a closer look at clinical data and discuss optimizing treatment for patients with DCB technology.

Join expert faculty as they guide you step-by-step through real-world AGENT Drug-Coated Balloon cases, offering clinical insights and practical strategies along the way.
Gain clarity and confidence in imaging while exploring lessons from industry leaders, interactive learning exercises, and real-life case studies.
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Coronary Drug-Coated Balloon (DCB) Reimbursement
AGENT DCB is a new and innovative technology that is supported by established coding for appropriate reimbursement.
Outpatient Services
Effective January 1, 2026, US Centers for Medicare & Medicaid Services (CMS) reassigned drug-coated balloon PCI procedures from APC 5192 to 5193 under the Hospital Outpatient Prospective Payment System (OPPS). 20
Transitional Pass-Through (TPT) Payment
- TPT provides additional reimbursement under the Hospital Outpatient Prospective Payment System (OPPS), effective January 1, 2025.21
- To receive the TPT payment, hospitals must report C9610 on outpatient claims for cases using AGENT DCB along with the code for the associated procedure.
Inpatient Services
New Technology Add-On Payment (NTAP)
- NTAP provides additional reimbursement under the CMS Inpatient Prospective Payment System (IPPS}, effective October 1, 2025.22
- To receive the NTAP, hospitals must report one of the ICD-10-PCS codes for AGENT DCB on inpatient claims (see the coding and billing guidance in the resources below).
The CPT Editorial Summary of Actions published May 16, 2025, confirms the transition of temporary CPT III codes for reporting DCB to permanent CPT I codes.
CPT*
- The Category Ill CPT codes for reporting DCB became effective January 1, 2025.
- The new Category I CPT codes for procedures involving AGENT DCB will take effect on January 1, 2027.
For questions regarding AGENT Drug-Coated Balloon reimbursement, please contact the Boston Scientific Reimbursement Support Line.
Email: IC.Reimbursement@bsci.com
Outpatient

AGENT DCB Outpatient Coding Guide
Guide listing outpatient CPT coding for outpatient PCI procedures using AGENT DCB.

AGENT DCB TPT Guide
Guide reviewing billing and coding information for Transitional Pass-Through (TPT) payment for AGENT DCB.

2025 AGENT DCB C-Code Guide
Quick reference guide discussing HCPCS C-Code reporting in the outpatient hospital setting.
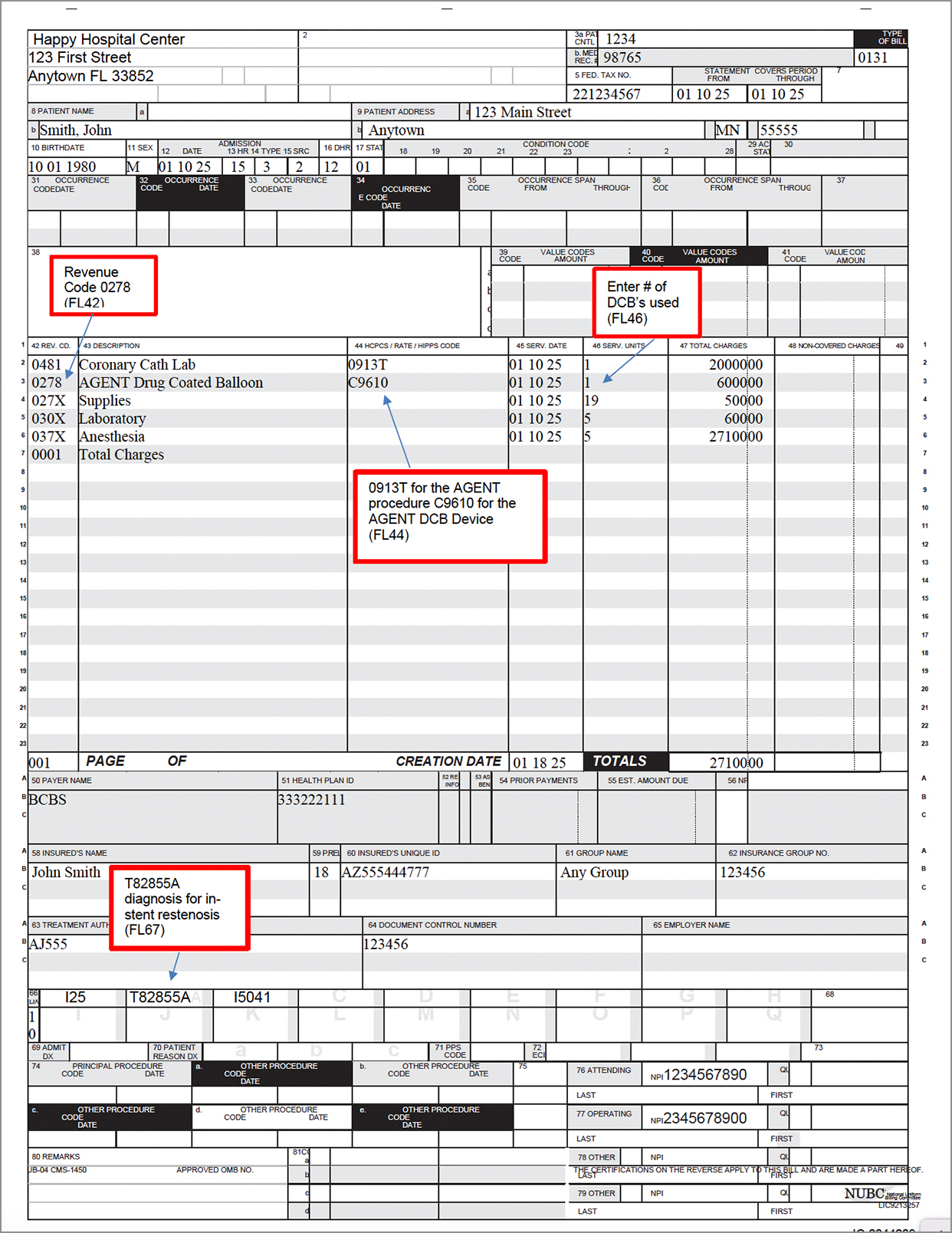
Sample Claim Form UB 04
Form to help with submitting hospital service reimbursement claims for AGENT DCB procedures.
Inpatient
Inpatient PCI with AGENT DCB Coding Guide
Guide listing inpatient ICD-10-PCS coding scenarios for inpatient PCI procedures using AGENT DCB.
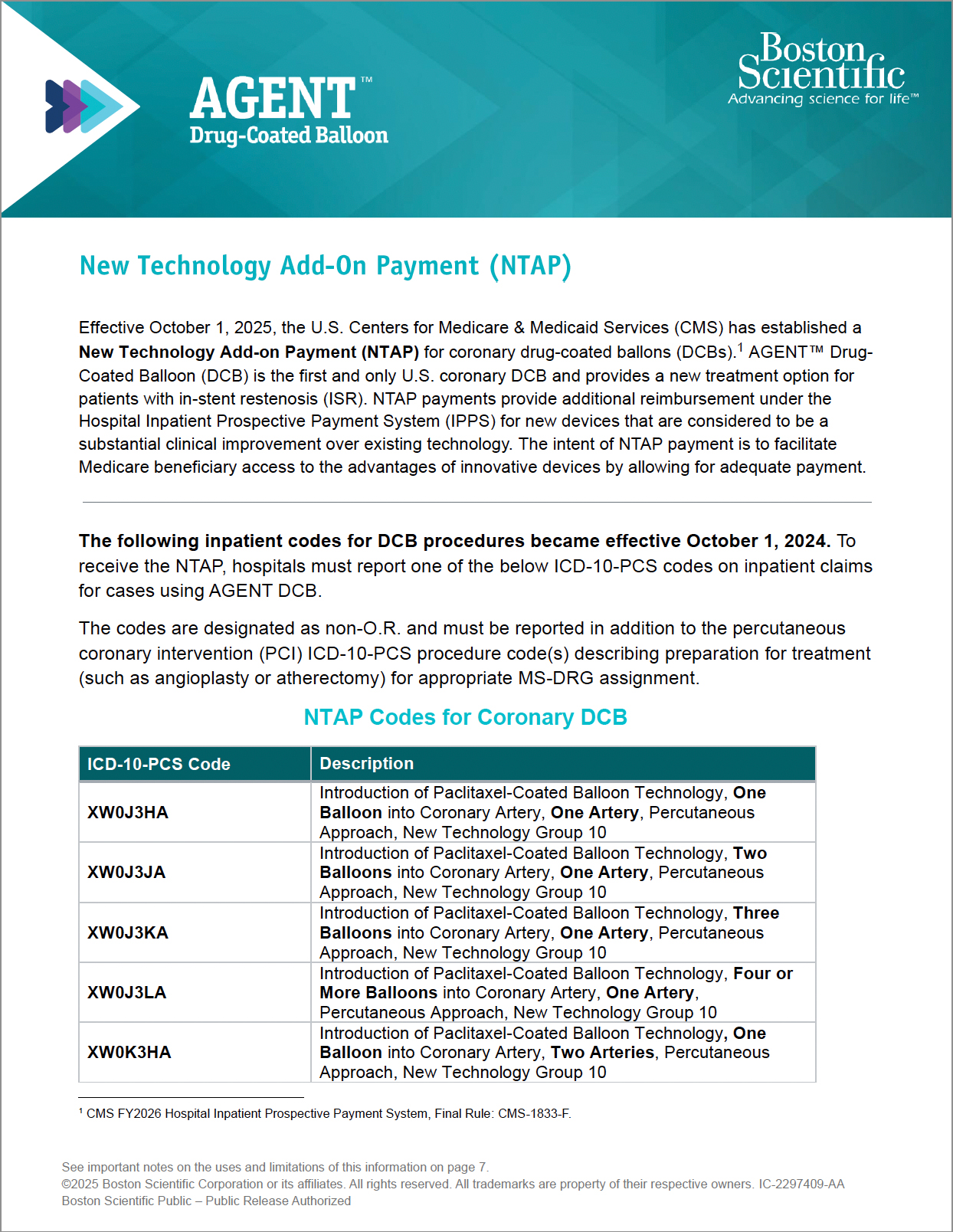
AGENT DCB NTAP Guide
Guide reviewing billing and coding information for New Technology Add-On Payment (NTAP) for AGENT DCB.

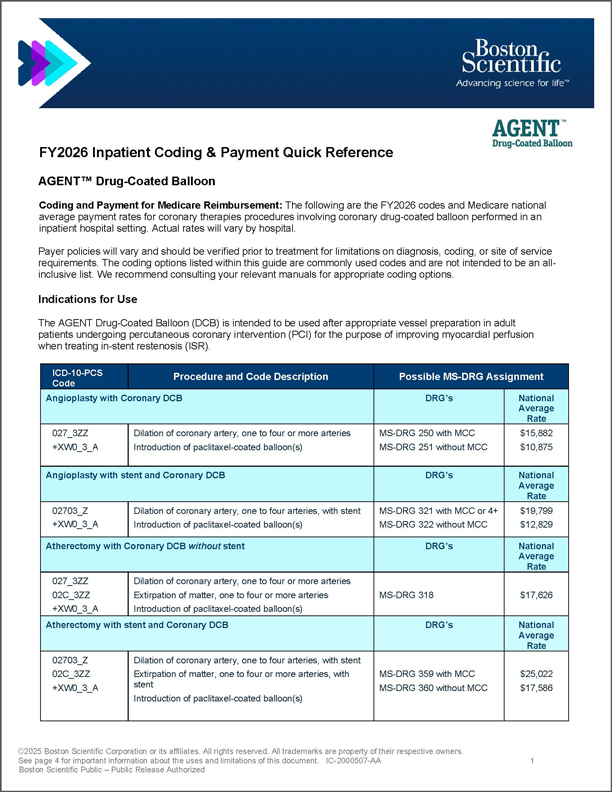
AGENT DCB Inpatient ICD-10-PCS Coding Guide
Quick reference guide highlighting current inpatient coding, MS-DRG assignments, and national average payment rates.
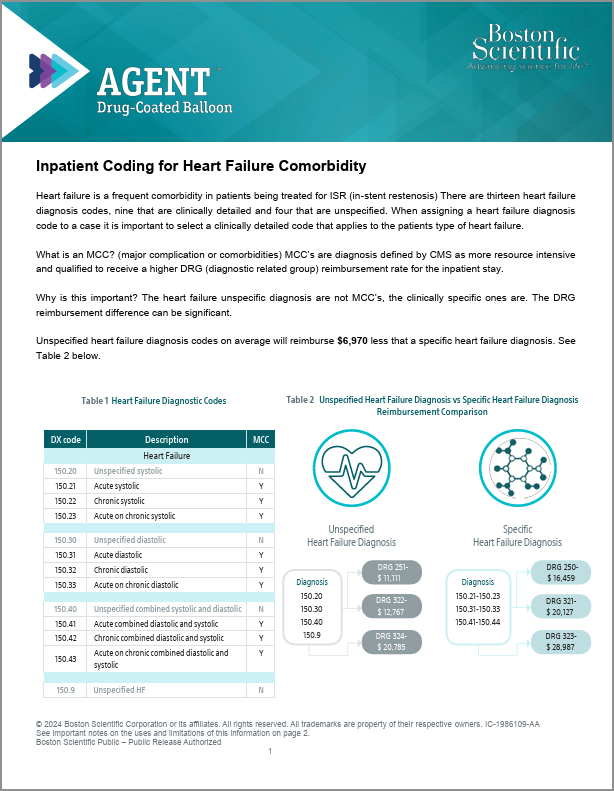
Heart Failure Coding Guide
Guide reviewing key information regarding accurately reporting heart failure diagnosis codes, why detailed reporting is important, and impacts to procedure payment.
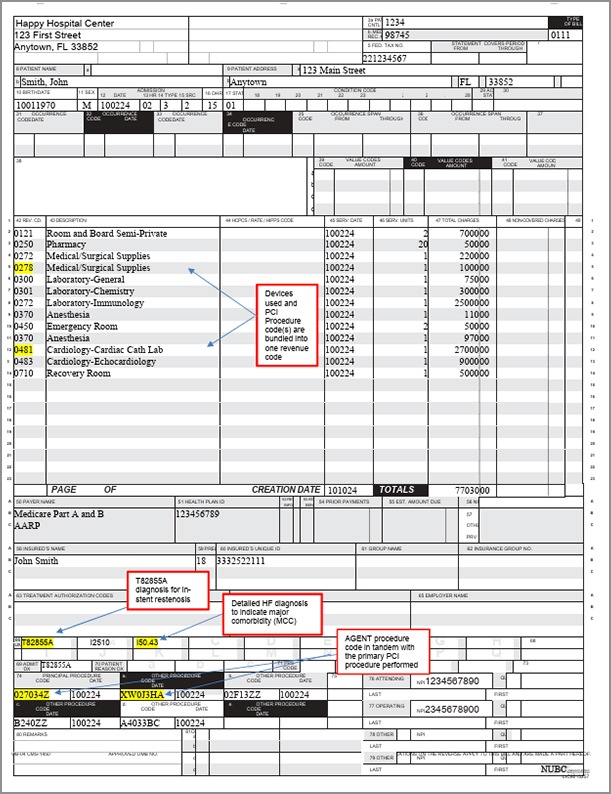
Sample Claim Form UB-04
Form to help with submitting hospital service reimbursement claims for AGENT DCB procedures.
Physician

AGENT DCB Physician Coding Guide
Guide listing physician CPT coding for PCI procedures using AGENT DCB.

Sample Claim Form 1500
Form to help with submitting physician service reimbursement claims for AGENT DCB procedures.
Additional resources for all sites of service
Quick reference guide discussing the ICD-10-CM diagnosis code reporting for in-stent restenosis (ISR).
CPT® 2025 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association.
These resources above are meant to be used by practicing physicians and allied healthcare professionals. These guides are not intended for patients or consumers.
Health economic and reimbursement information provided by Boston Scientific Corporation is gathered from third-party sources and is subject to change without notice as a result of complex and frequently changing laws, regulations, rules and policies. This information is presented for illustrative purposes only and does not constitute reimbursement or legal advice.
Resources
Physician Experience with DCBs and Patient Impact
Physician Experience with DCB Procedure: Imaging and Vessel Preparation
Patient Perspectives
1. Stolker JM, Cohen DJ, Kennedy KF, Pencina MJ, Lindsey JB, Mauri L, Cutlip DE, Kleiman NS. Evaluation of Drug-Eluting Stents and Ischemic Events (EVENT) Investigators. Repeat revascularization after contemporary percutaneous coronary intervention: An evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year
2. Moussa ID, Mohananey D, Saucedo J, et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76:1521–1531.
3. Kastrati A, Cassese S (2020). In-stent restenosis in the United States: Time to enrich its treatment armamentarium. In (Vol. 76, pp. 1532–1535): American College of Cardiology Foundation, Washington DC.
4. Tepe G, Brodmann M, Micari A, et al. 5-year outcomes of drug-coated balloons for peripheral artery in-stent restenosis, long lesions, and CTOs. JACC Cardiovasc Interv. 2023;16:1065–1078.
5. Kawamoto H, Ruparelia N, Latib A, et al. Drug-coated balloons versus second-generation drug-eluting stents for the management of recurrent multimetal-layered in-stent restenosis. JACC Cardiovasc Interv. 2015;8:1586–1594.
6. Yabushita H, Kawamoto H, Fujino Y, et al. Clinical outcomes of drug-eluting balloon for in-stent restenosis based on the number of metallic layers. Circ Cardiovasc Interv. 2018;11:e005935.
7. Market Data on file at BSC as of July 2023.
8. Nakamura M, Isawa T, Nakamura S, et al. Drug-coated balloon for the treatment of small vessel coronary artery disease – a randomized non-inferiority trial. Circ J. 2023;87:287–295.
9. Alfonso F, Coughlan JJ, Giacoppo D, Kastrati A, Byrne RA. Management of in-stent restenosis. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2022;18:e103–e123.10. Market Data on file at BSC as of July 2023.
10. Surapaneni M, et al. International Scholarly Research Notices 2012.
11. Kim M, et al. International journal of nanomedicine (2011): 6:2997–3009. Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process.
12. Teichgräber, U, et al. Head-to-head comparison of sirolimus- versus paclitaxel-coated balloon angioplasty in the femoropopliteal artery: study protocol for the randomized controlled SIRONA trial. Trials 22, 665 (2021)
13. Tzafriri A, et al. Journal of Controlled Release. 2019; 310:94–102. Taking paclitaxel coated balloons to a higher level: Predicting coating dissolution kinetics, tissue retention and dosing dynamics
14. Granada J. What’s next in drug-eluting SFA technology? Endovascular Today. 2017;16:9.
15. Data on file at BSC as of October 2025.
16. Yeh RW, Bachinsky W, Stoler R, et al. Rationale and design of a randomized study comparing the AGENT drug-coated balloon to plain old balloon angioplasty in patients with in-stent restenosis. Am Heart J. 2021;241:101–107.
17. AGENT IDE Clinical Trial data presented at CRT 2024 by Dr. Robert Yeh.
18. Data presented at TCT 2025 by Dr. Christina Lalani.
19. Data presented at TCT 2025 by Dr. Masato Nakamura. Results include over 1,300 Japanese patients treated with AGENT DCB (73.3% of the 1,800 PTX DCB patients).
20. CMS. FY 2026 OPPS Final Rule: CMS-1834-FC; Effective through December 31, 2026. https://www.cms.gov/medicare/payment/prospective-payment-systems/ambulatory-surgical-center-asc/asc-regulations-and-notices/cms-1834-fc
21. Medicare Hospital Outpatient Prospective Payment System (OPPS) Final Rule; CMS-1809-FC; p. 507; https://federalregister.gov/d/2024-25521.
22. Medicare Hospital Inpatient Prospective Payment System (IPPS) Final Rule; CMS-1833-F; p. 610; https://www.federalregister.gov/d/2025-14681.
Please review the AGENT Brief Summary for full instructions on use.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. The material not intended for use in France.
All trademarks are the property of their respective owners.