Boston Scientific accounts are for healthcare professionals only.
Introducing Endura Weight Loss Solutions
Patients living with obesity face many challenges on their weight loss journey – from making diet, exercise, and lifestyle changes to managing the ups and downs of their physical, mental, and emotional health. It’s a journey that requires real endurance.
Boston Scientific understands these challenges for your patients, and we offer a new approach to endobariatrics that provides innovative endoscopic options for physicians. Minimally invasive procedures. Proven, effective results. Endura Weight Loss Solutions. The future of weight loss is here.
For people living with obesity, lifestyle modifications and surgery have long been the only paths toward weight loss. Even with the growth of GLP-1-type medications, many patients have longed for another option. Dr. Brian Dunkin explains how endobariatric procedures – including endoscopic sleeve gastroplasty (ESG) – can help fill the gap.
1. Jirapinyo P, Kumar N, AlSamman MA, Thompson CC. Five-year outcomes of transoral outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest Endosc. 2019;91:1067–1073.
3. US FDA Pivotal Study. n = 137 Orbera, n = 136 control, p < 0.001. Most of the patients from the US FDA pivotal study were female and Caucasian. Because of this, data from this study may not accurately represent the same effectiveness and safety profile in Hispanic, African American, or other ethnic populations.
Hear from successful endobariatric teams
Dr. Christopher Chapman and nurse practitioner Sarah Kosinski share their experience establishing an endobariatric program.
Dr. Halim Charbel and registered dietitian Kaitlyn Cole explore the evolving landscape of endobariatrics.
Learn more about Endura Weight Loss Solutions
Download the Endura Weight Loss Solutions Frequently Asked Questions guide to learn more about this category of minimally invasive weight loss options for your patients. Get answers to common questions, patient insights, and training info – all in one place.
Insights from bariatric surgeons
Dr. Bob Snow shares insights from his 30-year career transitioning from open to laparoscopic and now endoscopic weight loss procedures.
Dr. John Tann explains how he integrates endoscopic weight loss procedures into his practice to expand treatment options for patients who may not want or qualify for surgery.

Learn about our Patient Education Call Center
Navigating weight loss options can be overwhelming. Our Patient Education Call Center can help patients understand their options for minimally invasive weight loss procedures – including endoscopic sleeve gastroplasty, intragastric balloon, and transoral outlet reduction.
Get updates


Hear from interventional gastroenterologists about their role in endobariatrics
Dr. Reem Sharaiha talks about how endoscopic weight loss procedures fit into today’s array of options for patients with obesity.
Dr. Andrew Storm talks about the role gastroenterologists play in addressing the obesity epidemic.
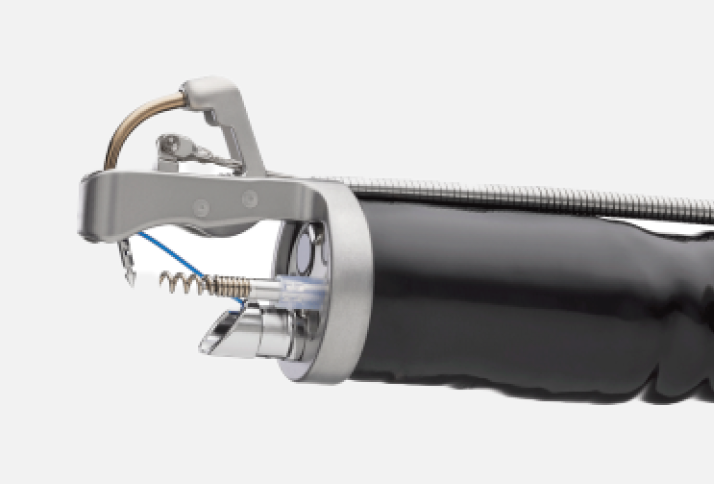
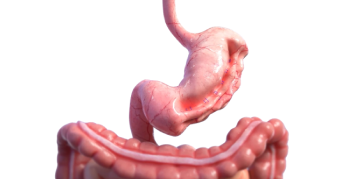
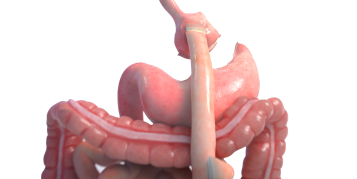
OverStitch™ Endoscopic Suturing System

OverStitch™ Endoscopic Suturing System

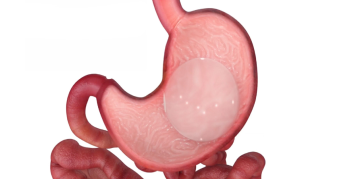
Orbera™ Intragastric Balloon System
Get updates on Endura Weight Loss Solutions
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
As a condition of the FDA De Novo Authorization of the OverStitch NXT and OverStitch Endoscopic Suturing System for endobariatric procedures (formerly referred to as the Apollo ESG and Apollo REVISE Systems), the devices should only be used for endoscopic sleeve gastroplasty (ESG) or to enable transoral outlet reduction (TORe) as a bariatric revision procedure by gastroenterologists and surgeons who have undergone specific training by the device manufacturer.
To fulfill the FDA requirement and special controls for these devices, Boston Scientific is required to independently host courses with consistent training curricula. More information regarding the referenced ESG and TORe revision procedure training courses is available through Boston Scientific.