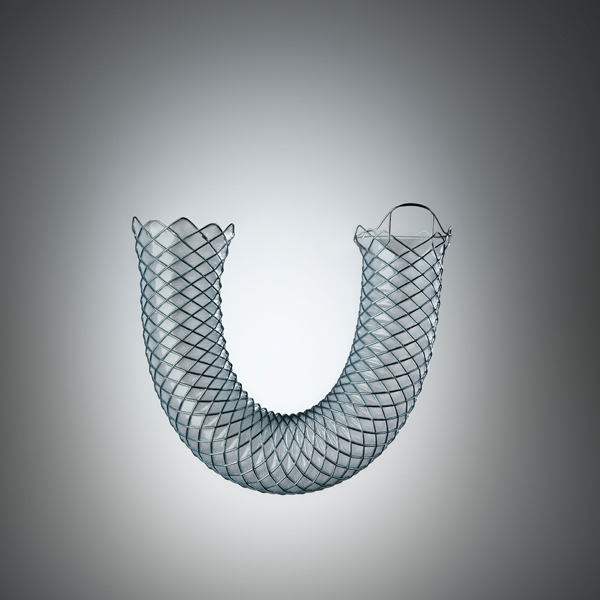
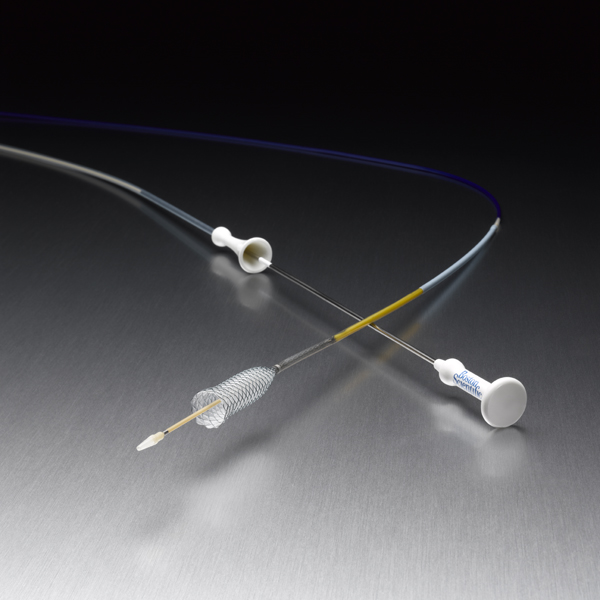
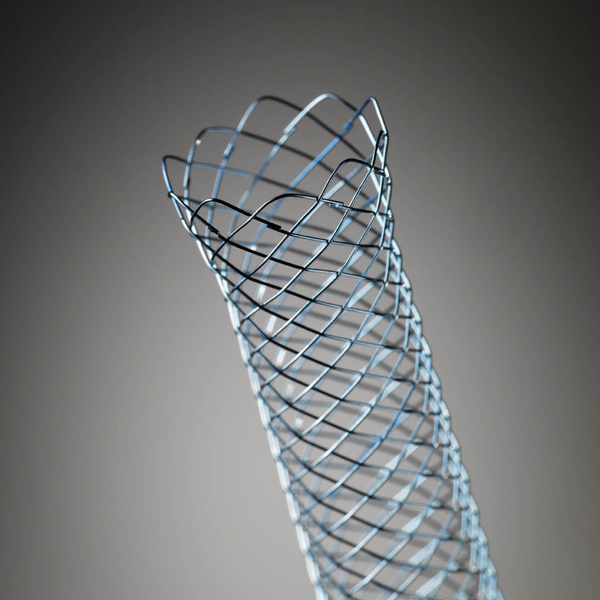
WallFlex™
Biliary RX Stents
The WallFlex Biliary RX Fully Covered Stent System RMV is currently the only biliary metal stent in the U.S. for indwell up to 12 months in the treatment of benign biliary strictures secondary to chronic pancreatitis.
Explore
Product Details
WallFlex Biliary RX Fully Covered RMV Stent
Removability
The fully covered RMV stent is indicated for indwell up to 12 months after placement in benign biliary strictures secondary to chronic pancreatitis.
Migration Resistance
Looped and flared stent ends are designed to help reduce the risk of tissue trauma and stent migration.
Tissue In-Growth Prevention
Closed cell construction and Permalume™ covered options designed to help resist tissue in-growth.1,2
Flexibility
Platinol™ Wire construction provides flexibility to help aid placement in tortuous anatomies.*
Radial Force
Radial force helps maintain stent patency and is designed to help reduce migration.
RX Biliary Delivery System
Stent Placement Accuracy
The RX biliary delivery system is reconstrainable up to 80% of deployment to aid in repositioning*** and is designed to facilitate physician control.
Endoscopic Placement
Closed cell construction and Permalume covered options designed to help resist tissue in-growth.1,2
Fluoroscopic Visualization
The four fluoroscopy markers and yellow transition zone are designed to help aid in stent placement accuracy when deployed using endoscopic visualization.
Stent Patency and Migration Satisfaction Program
Boston Scientific is offering a Patency and Anti-Migration Satisfaction Program for partially or fully covered Wallflex™ Stents. Please contact your territory manager to learn more.
Program Details
Boston Scientific will provide a free replacement WallFlex Stent of identical dimension if a partially or fully covered WallFlex Stent is implanted in accordance with the terms of the Program and the stent migrates or becomes occluded during the first year, and all other terms of the Program are fulfilled. This offer applies only to partially or fully covered WallFlex Stent endoscopically placed in accordance with the Directions for Use after January 1, 2023.
Replacement stents provided under the Program are not eligible for the Program. Claims under the Program are limited to one (1) new identical replacement stent. Stents provided pursuant to the Program will be accompanied by a no-charge invoice shipped directly to the hospital upon receipt of the appropriate documentation. The hospital is responsible for any reporting the replacement stent as a discount on the hospital’s cost report. This Program may not be available in all countries.
The Program is subject to modification or termination by Boston Scientific without prior notification. Contact your territory manager for details of the Program.
* Flexibility varies by size of stent
** In the event of incorrect positioning during the initial stent placement procedure, the partially covered and fully covered stent options may be removed using forceps to grasp the retrieval loop on the end of the stent. Warning: No warranty is made with regard to removability of this device by endoscopic means or otherwise.
Indications, contraindications, warnings and instructions for use can be found in the product labeling.
*** A stent cannot be reconstrained after the reconstrainment line has been exceeded
**** Endoscopic and fluoroscopic images courtesy of Adrian Hatfield, MD and Thomas Kowalski, MD
Ordering Information
WallFlex Biliary RX Stents
Fully covered, partially covered and uncovered WallFlex Biliary RX Stents are available in multiple sizes to accommodate different anatomical and clinical requirements. Below please find ordering information for WallFlex Biliary RX Stents. These stents may be used with short or long guidewires.

| Order Number | Diameter (mm) | Length (mm) | Covered Length (mm) (PC Only) | Catheter Diameter (Fr/mm) | Guidewire Diameter (in/mm) | Working Length (cm) | Packaging |
|---|---|---|---|---|---|---|---|
| M00570340 | 8 | 60 | – | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00570350 | 8 | 80 | – | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00547460** | 8 | 100 | – | 9.0 / 3.0 | .035 / .89 | 194 | Each |
| M00570360 | 10 | 40 | – | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00570370 | 10 | 60 | – | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00570380 | 10 | 80 | – | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00547480** | 10 | 100 | – | 9.0 / 3.0 | .035 / .89 | 194 | Each |
* Compatible with Boston Scientific .035" guidewires, including HydraJag JagwireTM High Performance Guidewire, DreamwireTM High Performance Guidewire, and JagwireTM Guidewire. Above (WallFlex Biliary RX Fully Covered Stent System) with PermalumeTM covering.
**Indicated for the palliative treatment of biliary strictures produced by malignant neoplasms and relief of malignant biliary obstruction prior to surgery.
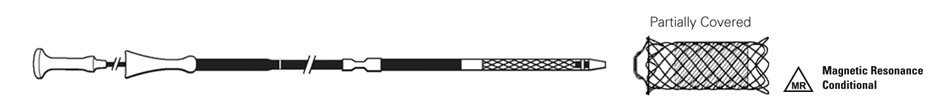
1 Through non-clinical testing, the WallFlex Biliary RX Stent has been shown to be MR Conditional (poses no known hazards under specified conditions) when used according to conditions described in the Directions for Use.
| Order Number | Diameter (mm) | Length (mm) | Covered Length (mm) (PC Only) | Catheter Diameter (Fr/mm) | Guidewire Diameter (in/mm) | Working Length (cm) | Packaging |
|---|---|---|---|---|---|---|---|
| M00570700 | 8 | 60 | 48 | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00570710 | 8 | 80 | 68 | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00547420 | 8 | 100 | 88 | 9.0 / 3.0 | .035 / .89 | 194 | Each |
| M00570720 | 10 | 40 | 28 | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00570730 | 10 | 60 | 48 | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00570740 | 10 | 80 | 68 | 8.5 / 2.83 | .035 / .89 | 194 | Each |
| M00547440 | 10 | 100 | 88 | 8.5 / 2.83 | .035 / .89 | 194 | Each |
* Compatible with Boston Scientific .035" guidewires, including HydraJag JagwireTM High Performance Guidewire, DreamwireTM High Performance Guidewire, and JagwireTM Guidewire. Above (WallFlex Biliary RX Partially Covered Stent System) with PermalumeTM covering.
1 Through non-clinical testing, the WallFlex Biliary RX Stent has been shown to be MR Conditional (poses no known hazards under specified conditions) when used according to conditions described in the Directions for Use.
| WallFlex Biliary RX Uncovered Stent System* | |||||||
|---|---|---|---|---|---|---|---|
| Order Number | Diameter (mm) | Length (mm) | Covered Length (mm) (PC Only) | Catheter Diameter (Fr/mm) | Guidewire Diameter (in/mm) | Working Length (cm) | Packaging |
| M00570600 | 8 | 40 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
| M00570610 | 8 | 60 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
| M00570620 | 8 | 80 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
| M00570630 | 8 | 100 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
| M00570890 | 10 | 40 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
| M00570640 | 10 | 60 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
| M00570650 | 10 | 80 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
| M00570660 | 10 | 100 | – | 8.0 / 2.67 | .035 / .89 | 194 | Each |
* Compatible with Boston Scientific .035" guidewires, including HydraJag JagwireTM High Performance Guidewire, DreamwireTM High Performance Guidewire, and JagwireTM Guidewire.
1 Through non-clinical testing, the WallFlex Biliary RX Stent has been shown to be MR Conditional (poses no known hazards under specified conditions) when used according to conditions described in the Directions for Use.
Education and Training
Explore online and in-person resources to advance your skills and manage your practice with physician-led videos, specialized training, and reimbursement tools.
Reimbursement
Physician & Patient Resources
REFERENCES
- Moss A.; Morris E.; MacMathuna P.; Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Systematic Review, 25 Jan. 2006.
- Soderlund K., Linder S.; Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointestinal Endoscopy, 2006; 63: 986-995.
Caution: U.S. Federal law restricts these devices to sale by or on the order of a physician.
INDICATIONS FOR USE in the United States:
The WallFlex Biliary RX Fully Covered Stent System RMV is indicated for use in the palliative treatment of biliary strictures produced by malignant neoplasms, relief of malignant biliary obstruction prior to surgery and for indwell up to 12 months in the treatment of benign biliary strictures secondary to chronic pancreatitis.
WallFlex Biliary RX Stent System Indications: indicated for use in the palliative treatment of biliary strictures produced by malignant neoplasms and relief of malignant biliary obstruction prior to surgery.
WARNINGS:
The safety and effectiveness of the stent has not been established for indwell periods exceeding 12 months. The WallFlex Biliary RX Fully Covered Stent System RMV is for single-use only. The safety and effectiveness of the WallFlex Biliary RX Fully Covered Stent System RMV for use in the vascular system has not been established.
The safety and effectiveness of the WallFlex Biliary RX Fully Covered Stent System RMV has not been established in the treatment of benign biliary anastomotic strictures in liver transplant patients and benign biliary post abdominal surgery strictures. Testing of overlapped stents has not been conducted. The stent contains nickel, which may cause an allergic reaction in individuals with nickel sensitivity.
Warning: The safety and effectiveness of this device for use in vascular system has not been established.
LIMITATIONS:
The sale, distribution, and use of the device are restricted to prescription use in accordance with 21 CFR §801.109
CONTRAINDICTIONS:
The WallFlex Biliary RX Fully Covered Stent should not be placed in strictures that cannot be dilated enough to pass the delivery system, in a perforated duct, or in very small intrahepatic ducts.
The WallFlex Biliary RX Fully Covered Stent System RMV should not be used in patients for whom endoscopic techniques are contraindicated.
PLEASE REFER TO THE LABELING FOR A MORE COMPLETE LIST OF WARNINGS, PRECAUTIONS AND CONTRAINDICATIONS