Boston Scientific accounts are for healthcare professionals only.
Indications, safety, and warnings
LUX-Dx II™ and LUX-Dx II+™ Insertable Cardiac Monitor Systems
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a licensed practitioner. Prior to use, please refer to all applicable “Instructions for Use” for more information on Intended Use/Indications for Use, Contraindications, Warnings, Precautions, Potential Adverse Events, and Operator’s Instructions.
INTENDED USE/INDICATIONS FOR USE
The LUX-Dx Insertable Cardiac Monitor (ICM) is intended to monitor and record subcutaneous electrocardiogram (SECG). The recorded S-ECG is used for the clinical evaluation and diagnosis of cardiac arrhythmias. The LUX-Dx is indicated for use in patients that have a known heart condition and are at risk of developing an abnormal heart
rhythm, or have symptoms that may suggest a cardiac arrhythmia such as dizziness, palpitations, syncope, chest pain, and/or shortness of breath. The LUX-Dx ICM is indicated for atrial fibrillation monitoring in patients that have been previously diagnosed or treated for atrial fibrillation.
The LUX-Dx has not been tested specifically for pediatric use.
CONTRAINDICATIONS
There are no known contraindications for the insertion of the LUX-Dx insertable cardiac monitor. However, the patient’s particular medical condition may dictate whether or not they can tolerate a subcutaneous, chronically-inserted device.
LATITUDE Clarity is contraindicated for use with any device other than a compatible Boston Scientific device.
WARNINGS
General
Co-implanted device interaction. Concomitant use of the ICM system and implanted electro-mechanical devices [for example implantable neuromodulation/neurostimulation systems, ventricular assist device (VAD), or implantable insulin pump or drug pump] can result in interactions that could compromise the function of the ICM, the co-implanted device, or both. Electromagnetic interference (EMI) or therapy delivery from the co-implanted device can interfere with ICM sensing and/or rate assessment, resulting in failure to monitor or record when needed. Verify sensing configuration, operation modes, surgical considerations and existing placement of all involved devices prior to any co-implant. To help prevent undesirable interactions, test the ICM system when used in combination with the co-implanted device. Following completion of the interaction testing, thorough follow-up evaluation of all co-implanted devices should be performed to ensure that device functions have not been compromised. If operational settings of the co-implanted devices change or if patient conditions change which may affect ICM sensing, re-evaluation of the co-implanted devices may be required.
Labeling knowledge. Read this manual thoroughly before using the ICM system to avoid damage to the device. Such damage can result in patient injury or death.
For single patient use only. Do not reuse, reprocess, or resterilize the insertable cardiac monitor or insertion tools. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or lead to device failure which, in turn, may result in patient injury, illness, or death. Reuse, reprocessing, or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient. The medical professional may reposition or re-insert the device within a single procedure.
Sharp object. Incision tool is sharp. Take precautions to ensure that it is handled properly. Dispose of incision tool directly into a sharps disposal container labeled with a biological hazard symbol. Sharps waste should be safely disposed of using available sharps waste channels in accordance with hospital, administrative, and/or local government policy.
Insertion
Tunneling. The insertion tool is intended to be used in the subcutaneous space. Always be aware of the location of the tool tip relative to the patient anatomy. Hold the insertion tool at a narrow angle while tunneling. Unintended tissue damage may result if the device is inserted at a large angle.
Incision tool blade placement. Always be aware of the location of the incision tool blade relative to the patient anatomy. Unintended tissue damage may result if the incision tool is inserted beyond the blade.
Post Insertion
Diathermy. Do not expose a patient with an ICM system to diathermy. The interaction of diathermy therapy with an insertable cardiac monitor can damage the device and cause patient injury.
Firmware update must be completed. Once a firmware update begins, the patient will not be monitored until the update is completed. If the firmware update is skipped, the patient is still monitored.
Interrogate device, save data, and check device function. The influence of medical equipment on implanted devices varies considerably according to the type of unit and energy levels employed. In situations where the risks are known, always interrogate the device and save data before the procedure, and check device function afterwards.
Magnet compatibility. Magnet model 6386 has been tested for use with the ICM system. Use of any other magnets has not been tested and could result in failure to initiate communication with the device.
Magnet use. The magnet provided with the ICM system may cause interference with devices sensitive to magnetic fields such as hearing aids, pacemakers, and other implanted devices. It can also permanently disable some magnetic strip cards. Keep the magnet at least 15 cm (6 inches) away from items sensitive to magnetic fields, including the ICM device when the magnet is not being used to initiate communication between the device and the clinic or patient app.
Mobile devices and magnet are MR Unsafe. The mobile devices and magnet are MR Unsafe and must remain outside the MRI site Zone III (and higher) as defined by the American College of Radiology Guidance Document on MR Safe Practices1. Under no circumstances should the mobile device or magnet be brought into the MRI scanner room, the control room, or the MRI site Zone III or IV areas.
MR conditional requirements. Unless all of the MRI Conditions of Use are met, MRI scanning of the patient does not meet MR Conditional requirements for the inserted device, and significant harm to or death of the patient and/or damage to the inserted device may result.
Scanning with other devices. Scanning patients who have other MR Conditional devices is acceptable if all the MR Conditional requirements for each of the implanted devices are met. Do not conduct an MRI scan if any conditions or implants prohibit it.
Protected environments. Advise patients to seek medical guidance before entering environments that could adversely affect the operation of the active implantable medical device, including areas protected by a warning notice that prevents entry by patients.
PRECAUTIONS
General
Not a medical alert system. The ICM system is not meant to assist with health emergencies. Patients who need immediate medical attention, should call their health care provider or seek emergency provider medical services.
FCC Compliance. Changes or modifications not expressly approved by Boston Scientific could void the user’s authority to operate the equipment.
Patient Settings. Ensure that each patient's alert configuration and programming settings are appropriate when the patient is enrolled and, if applicable, after the device is replaced.
Clinical Considerations
Check device battery before insertion. Check the device battery prior to insertion. If a low battery condition isindicated, do not insert the device.
Device does not alert patient. The device cannot alert the patient to emergency situations.
Magnet use impact on longevity. Use of the magnet to initiate communication has been accounted for in the projected battery life of the inserted device. Using this feature more often than directed may lead to a decrease in the battery life of the device.
Sterilization and Storage
If package is damaged. The blister trays and contents are sterilized with ethylene oxide gas before final packaging. When the device is received, it is sterile provided the container is intact. If the packaging is wet, punctured, opened, or otherwise damaged, return the device to Boston Scientific.
If device is dropped. Do not insert a device which has been dropped while outside of its intact shelf package. Do not insert a device which has been dropped from a height of more than 24 inches (61 cm) while within its intact shelf package. Sterility, integrity, and/or function cannot be guaranteed under these conditions, and the device should be returned to Boston Scientific for inspection.
Mobile device and magnet. The mobile device and magnet are non-sterile. Do not sterilize the mobile device or magnet. These items must be contained in a sterile barrier before use in the sterile field.
Use by date. Insert the device before or on the USE BY date on the package label because this date reflects a validated shelf life. For example, if the date is January 1, do not insert on or after January 2.
System protection. Store mobile devices containing Boston Scientific mobile applications in a secure location when not in use and take appropriate measures to prevent theft or unauthorized access.
Device storage. Store the insertable cardiac monitor in a clean area away from unshielded magnets, kits containing unshielded magnets, and sources of EMI to avoid device damage.
Magnet storage. Store the magnet between -20 to 70° C (-4 to 158° F) to avoid risk of de-magnetization or damage to the magnet case.
Device storage temperature and equilibrium. Recommended storage temperatures are 0°C–50°C (32°F–122° F). Allow the device to reach an ambient temperature before using telemetry communication capabilities, programming, or inserting the device because temperature extremes may affect initial device function.
Insertion
Breast implant rupture or leaking. Use extra care in patients with breast implants when inserting an ICM device. Breast implant rupture or leaking may result if the breast implant is damaged during the ICM insertion procedure.
Mobile device battery. Verify that the mobile device is sufficiently charged for the implant procedure; if not sufficiently charged, difficulty with set-up and system initialization may occur.
Expected benefits. Determine whether the expected device benefits provided by programmable options outweigh the possibility of more rapid battery depletion.
Evaluate patient for surgery. There may be additional factors regarding the patient's overall health and medical condition that, while not related to device function or purpose, could render the patient a poor candidate for insertion of this system. Cardiac health advocacy groups may have published guidelines that may be helpful in conducting this evaluation.
Handling the device. Use caution when using medical surgical instruments to remove the device during a repositioning as they could damage the device.
Magnet
Magnet replacement. If you lose your magnet or if the plastic coating surrounding the magnet cracks, contact Boston Scientific for a replacement.
Magnet cleaning. Clean your magnet with only the following cleaning agents as needed to avoid risk of damage to the magnet case: warm tap water, Isopropyl alcohol (70% concentration), or a weak concentration of mild detergent mixed with warm water. Apply cleaning agents to a soft cloth and use gentle pressure to avoid damaging the markings. Do not submerge the magnet or place under running water to prevent risk of water getting inside the magnet case.
Device Programming
Device communication. Use only the clinic or patient app to communicate with this device.
Sensing adjustment. Following any adjustments to sensing parameters, always verify appropriate sensing. Programming Sensitivity to the highest value (lowest sensitivity) may result in delayed detection or undersensing of cardiac activity. Likewise, programming to the lowest value (highest sensitivity) may result in oversensing of non-cardiac signals.
Patient app use restriction. The patient app is paired with a patient’s specific inserted device. It will not work with other inserted devices and should only be used as directed by the patient’s healthcare provider. Unauthorized use could interrupt the prescribed operation of the device.
Environmental and Medical Hazards
- Avoid electromagnetic interference (EMI). Advise patients to avoid sources of EMI, including but not limited to radio frequency interference (RFI) from wireless electronic products, which may have the potential to temporarily interfere with the device’s ability to detect and monitor the patient’s heart rate. It could also delay or prolong communication between the device and the patient or clinic apps. Moving away from the source of the EMI or turning off the source usually allows the sensor device to return to normal operation.
Examples of potential EMI sources are:- Electrical power sources, arc welding or resistance welding equipment, and robotic jacks
- High voltage power distribution lines
- Electrical smelting furnaces
- Large RF transmitters such as radar
- Radio transmitters, including those used to control toys
- Electronic surveillance (antitheft) devices
- An alternator on a car that is running
- Medical treatments and diagnostic tests in which an electrical current is passed through the body, such as TENS, electrocautery, electrolysis/thermolysis, electrodiagnostic testing, electromyography, or nerve conduction studies
- Any externally applied device that uses an automatic lead detection alarm system (e.g., an EKG machine)
- External defibrillation. It can take a few seconds for sensing to recover after an external shock is delivered.
- External defibrillation or cardioversion can damage the device. To help prevent damage to the device, consider the following:
- Avoid placing a pad (or paddle) directly over the device. Position the pads (or paddles) as far from the device as possible.
- Set energy output of external defibrillation equipment as low as clinically acceptable. Following external cardioversion or defibrillation, verify device function.
- Cardiopulmonary resuscitation. Cardiopulmonary resuscitation (CPR) may temporarily interfere with sensing and may damage the device.
- Electrical interference. Hospital medical equipment or equipment in home or occupational environments may cause damage to the device or could lead to oversensing. To reduce the potential of damage, ensure there is minimum separation between the device and other medical equipment and verify operation of the device following any patient procedure. Examples of equipment are as follows:
- Diathermy
- High levels of radiation
- Electrosurgical cautery
- Lithotripsy
- Ultrasound
- Radio frequency ablation
- Electronic article surveillance (EAS)
- Ionizing radiation therapy. It is not possible to specify a safe radiation dosage or guarantee proper device function following exposure to ionizing radiation. Multiple factors collectively determine the impact of radiation therapy on an inserted device, including proximity of the device to the radiation beam, type and energy level of the radiation beam, dose rate, total dose delivered over the life of the device, and shielding of the device. The impact of ionizing radiation will also vary from one device to another and may range from no changes in function to a loss of monitoring.
Sources of ionizing radiation vary significantly in their potential impact on an inserted device.
Several therapeutic radiation sources are capable of interfering with or damaging an inserted device, including those used for the treatment of cancer, such as radioactive cobalt, linear accelerators, radioactive seeds, and betatrons.
Prior to a course of therapeutic radiation treatment, the patient’s radiation oncologist and cardiologist or electrophysiologist should consider all patient management options, including increased follow-up and device replacement. Other considerations include:- Shielding the device with a radiation-resistant material, regardless of the distance between the device and the radiation beam.
- Determining the appropriate level of patient monitoring during treatment.
Evaluate device operation during and following the course of radiation treatment to exercise as much device functionality as possible. The extent, timing, and frequency of this evaluation relative to the radiation therapy regimen are dependent upon current patient health, and therefore should be determined by the attending cardiologist or electrophysiologist.
The effects of radiation exposure on the implanted device may remain undetected until some time following exposure. For this reason, continue to monitor device function closely and use caution when programming a feature in the weeks or months following radiation therapy.
- Electrocautery and radio frequency (RF) ablation. If electrocautery or RF ablation is medically necessary, observe the following to minimize risk to the patient and device:
- Avoid direct contact between the electrocautery equipment or ablation catheters and the device
- Keep the path of the electrical current as far away as possible from the device.
- If RF ablation and/or electrocautery is performed on tissue near the device, verify device function.
- For electrocautery, use a bipolar electrocautery system where possible and use short, intermittent, and irregular bursts at the lowest feasible energy levels.
Lithotripsy. Extracorporeal shock wave lithotripsy (ESWL) may cause electromagnetic interference with or damage to the device. If ESWL is medically necessary, focus the ESWL beam at least 15 cm (6 in) away from the device to minimize the potential for encountering interaction.
Ultrasound energy. Therapeutic ultrasound (e.g., lithotripsy) energy may damage the device. If therapeutic ultrasound energy must be used, avoid focusing near the insertion site. Diagnostic ultrasound (e.g., echocardiography) is not known to be harmful to the device.
Conducted electrical current. Any medical equipment, treatment, therapy, or diagnostic test that introduces electrical current into the patient has the potential to interfere with device function. Medical therapies, treatments, and diagnostic tests that use conducted electrical current (e.g., TENS, electrocautery, electrolysis/thermolysis, electrodiagnostic testing, electromyography, or nerve conduction studies) may interfere with or damage the device. After the treatment, verify device function.
- Transcutaneous electrical nerve stimulation (TENS). TENS involves passing electrical current through the body, and may interfere with device function. If TENS is medically necessary, evaluate the TENS therapy settings for compatibility with the device. The following guidelines may reduce the likelihood of interaction.
- Place the TENS electrodes as close together and as far away from the device as possible.
- Use the lowest clinically-appropriate TENS energy output.
- Additional steps can be taken to help reduce interference during in-clinic use of TENS:
- If interference is suspected during in-clinic use, turn off the TENS unit. – Do not change TENS settings until you have verified that the new settings do not interfere with device function.
- If TENS is medically necessary outside the clinical setting (at-home use), provide patients with the following instructions:
- Do not change the TENS settings or electrode positions unless instructed to do so.
- End each TENS session by turning off the unit before removing the electrodes.
- Magnetic fields. Advise patients to avoid extended exposure to strong (greater than 10 gauss or 1 mTesla) magnetic fields. Examples of magnetic sources include:
- Industrial transformers and motors
- Large stereo speakers
- Telephone receivers if held within 1.27 cm (0.5 inches) of the device
- Magnetic wands such as those used for airport security
Elevated pressures. The International Standards Organization (ISO) has not approved a standardized pressure test for implantable devices that experience hyperbaric oxygen therapy (HBOT) or SCUBA diving. However, Boston Scientific developed a test protocol to evaluate device performance upon exposure to elevated atmospheric pressures. The following summary of pressure testing should not be viewed as and is not an endorsement of HBOT or SCUBA diving.
Elevated pressures due to HBOT or SCUBA diving may damage the device. During laboratory testing, all devices in the test sample functioned as designed when exposed to more than 200 cycles at a pressure up to 3.0 ATA. Laboratory testing did not characterize the impact of elevated pressure on device performance or physiological response while implanted in a human body.
Pressure for each test cycle began at ambient/room pressure, increased to a high pressure level, and then returned to ambient pressure. Although dwell time (the amount of time under elevated pressure) may have an impact on human physiology, testing indicated it did not impact device performance. Pressure value equivalencies are provided in the following table.
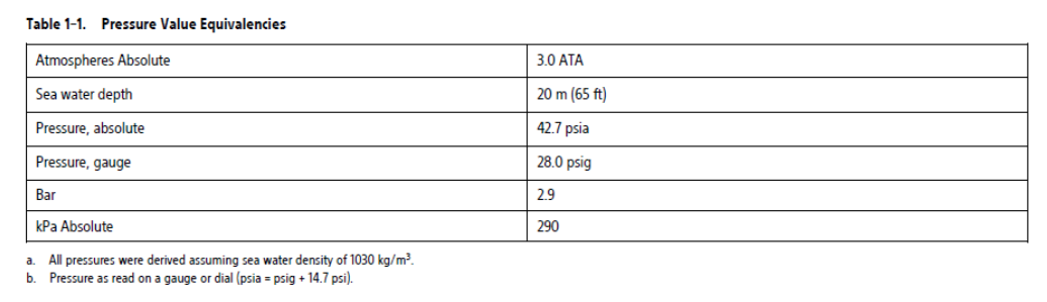
More frequent device follow-up may be warranted in conjunction with HBOT or SCUBA diving. Evaluate device operation following high pressure exposure. The extent, timing, and frequency of this evaluation relative to the high pressure exposure are dependent upon current patient health and should be determined by the attending health care provider. If you have additional questions, or would like more detail regarding the test protocol or test results specific to HBOT or SCUBA diving, contact Boston Scientific using the information on the back cover.
Mammography procedure. During a mammography procedure, manipulation or angular stress of the device between the plates could cause tissue trauma, vascular trauma, or pain, or affect device sensing. Before scheduling a mammogram, the health care provider should weigh the potential risks against the benefits and evaluate other diagnostic options. Allow sufficient time for the incision to heal before performing a mammography procedure.
Follow-up
Follow-up considerations for patients leaving the country. Patients can travel globally and still be followed remotely by their primary clinician if there is a Wi-Fi or cellular connection to the patient app. There are countries that will not be able to provide in-clinic follow-up services. Contact Boston Scientific, using the information on the back cover, for help in determining feasibility of device follow-up in the patient's destination country.
Device Removal and Disposal
- Handling at time of disposal. Clean and disinfect the device using standard biohazard handling techniques.
- Incineration. Remove the device before cremation and incineration as temperatures might cause the device to explode.
POTENTIAL ADVERSE EVENTS
Insertion and usage of this product may result in adverse events which may lead to injury, death, or other serious adverse reactions. If any adverse events occur, invasive corrective action and/or ICM system modification or removal may be required.
Potential adverse events related to insertion of the device may include, but are not limited to, the following:
Device migration
Erosion
Foreign body rejection phenomena
Formation of hematomas or seromas
Infection
Local tissue reaction
Tissue damage
Potential adverse events related to device operation may include, but are not limited to, the following:
Premature battery depletion
Sensing issues
Error codes
Loss of telemetry
Transient procedural adverse events are expected in some patients. These include, but are not limited to discomfort, pain, anxiety, and other systemic symptoms that might be related to medications or other interventions performed during implant.
For a list of potential adverse events associated with MRI scanning, refer to the MRI Technical Guide at www.bostonscientific-elabeling.com.
Any serious incident that occurs in relation to this device should be reported to Boston Scientific and to the relevant local regulatory authority.
97104968 (Rev. B)