Are you a patient looking to learn more about the Intracept Procedure? Visit our Intracept patient site.
System overview
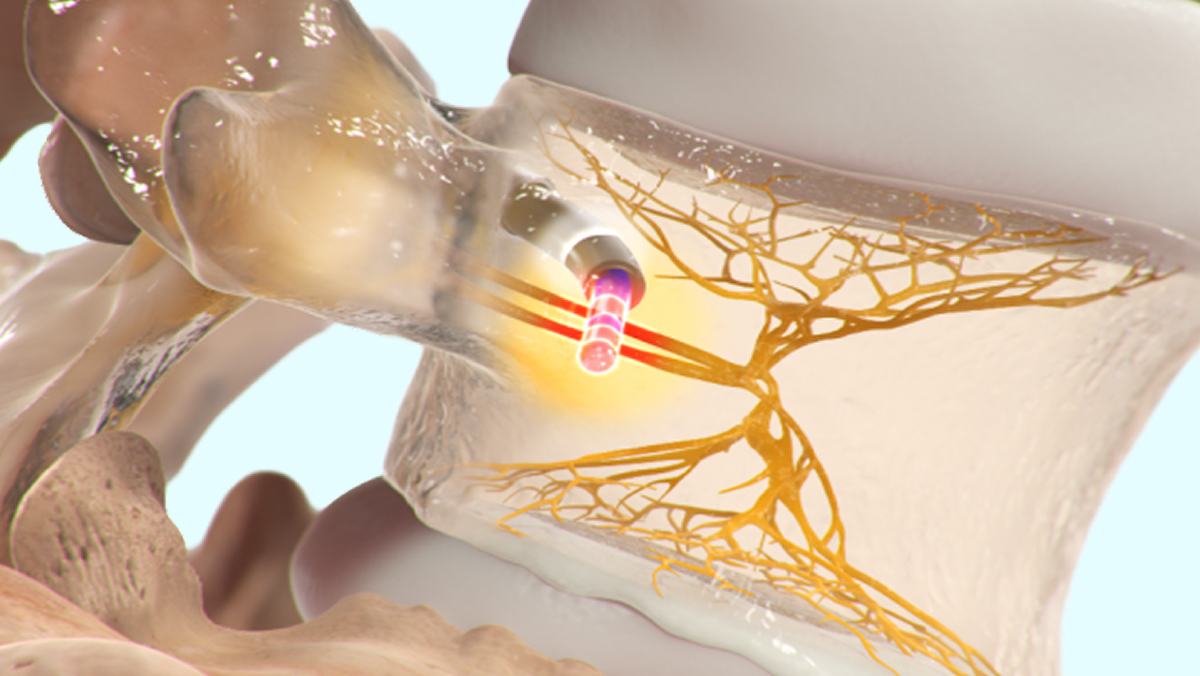
The Intracept System enables a physician to effectively target and ablate the basivertebral nerve (BVN) to provide relief of vertebrogenic chronic low back pain (CLBP). The Intracept System includes purpose-built Intracept Access Instruments for creating a path to the BVN, proprietary radiofrequency (RF) ablation technology for effectively ablating the BVN, and comprehensive training and case support.