Boston Scientific accounts are for healthcare professionals only.

Express™ LD Iliac and Biliary Premounted Stent System
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical Data
- Training
- Resources
Compression resistance and conformability
Only Express LD Iliac and Biliary Stent was engineered to provide the perfect balance of compression resistance and conformability—without trade-off.

How it works
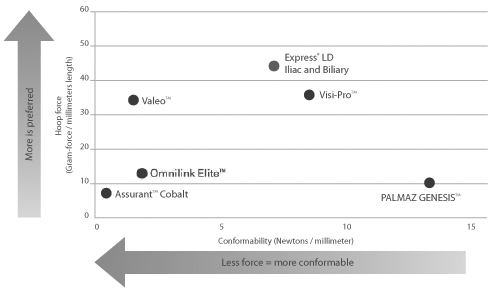
Compression resistance and conformability are crucial stent attributes that help improve vessel patency within tortuous vasculature.
Compression Resistance measured as the force required to compress the fully expanded stent. Compression Resistance measured using a hoop force tester in 37° air. Conformability measured by torque required to achieve a constant curvature target as measured by angular deflection. Bench testing performed by Boston Scientific Corporation. Data on file. N = 3. Express LD tested: 8 x 37 mm. Visi-Pro tested: 8 x 37 mm. Palmaz Genesis tested: 8 x 39 mm. Omnilink Elite tested: 8 x 39 mm. Valeo tested: 8 x 36 mm. Assurant tested: 8 x 40 mm. Bench test results may not necessarily be indicative of clinical performance.
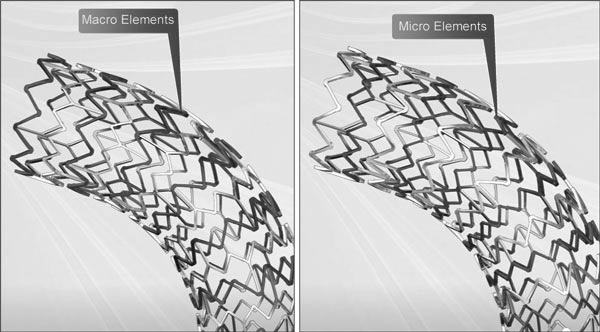
Why choose Express LD
Express LD Stent System features a patented Tandem Architecture™ Stent Design
Featured clinical data
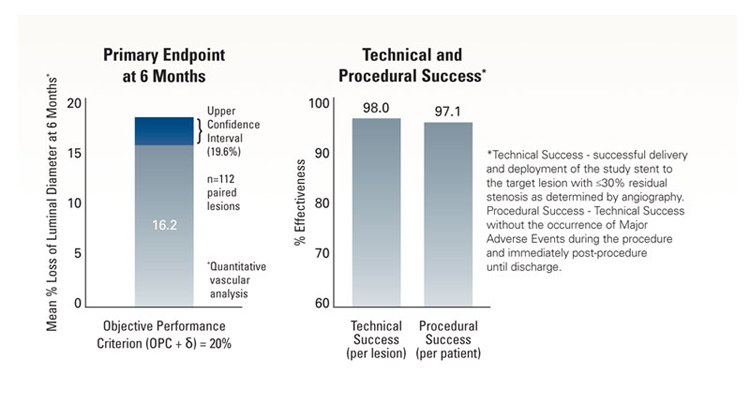
MELODIE Trial* Summary
MELODIE is a prospective, multi-center, single arm study to obtain additional data on the safety and efficacy of the Express™ Vascular LD stent implantation in the treatment of stenosed or occlusive atherosclerotic disease (de novo or restenotic) in iliac arteries (common or external).
MELODIE Trial Objective
The objective was to demonstrate non-inferiority of the Express™ LD Stent for the treatment of atherosclerotic iliac artery lesions as compared to an objective performance criterion* (OPC), with a primary endpoint of mean percent luminal diameter loss at 6 months.
*Note: the OPC=15%, δ=5%.
Primary Endpoint
Definition
- Angiographic mean percent loss of luminal diameter at 6 months post-procedure, defined as [POST MLD1 - FUP MLD2 / POST MLD] x 100
References:
1. Post MLD = Post-procedure minimum lumen diameter.
2. FUP MLD = Follow-up minimum lumen diameter at 6 months.
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Resources list
Express is a registered or unregistered trademark of Boston Scientific Corporation or its affiliates. Valeo is a registered trademark of C. R. Bard Corporation. Omnilink Elite is a trademark of Abbott Laboratories. Assurant Cobalt is a registered trademark of Medtronic Corporation. PALMAZ GENESIS is a registered trademark of Cordis Corporation. Visi-Pro is a registered trademark of ev3 Corporation.
*Stockx L, Poncyljusz W, Krzanowski M, et al. Express LD vascular stent in the treatment of iliac artery lesions: 24-month results from the MELODIE trial. J Endovasc Ther. 2010;17(5):633-641. doi:10.1583/09-2917MR.1




