Boston Scientific accounts are for healthcare professionals only.




Ultra Retropubic Mid-Urethral Sling Family
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Training
- Resources
- Ordering information
Fueled by physician insights and feedback
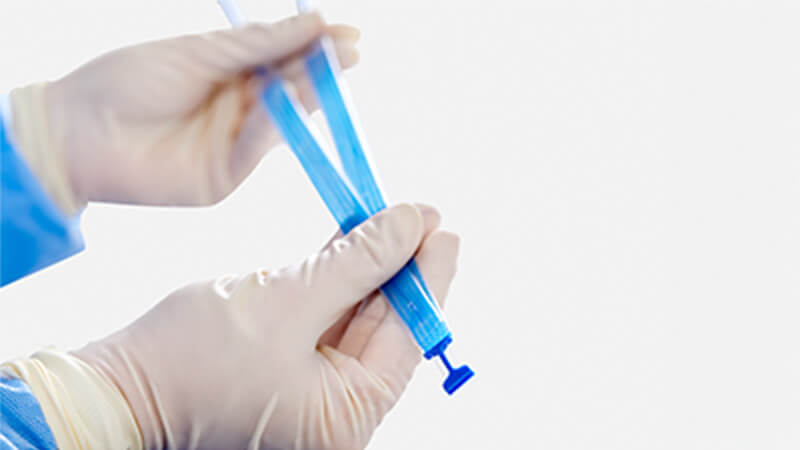
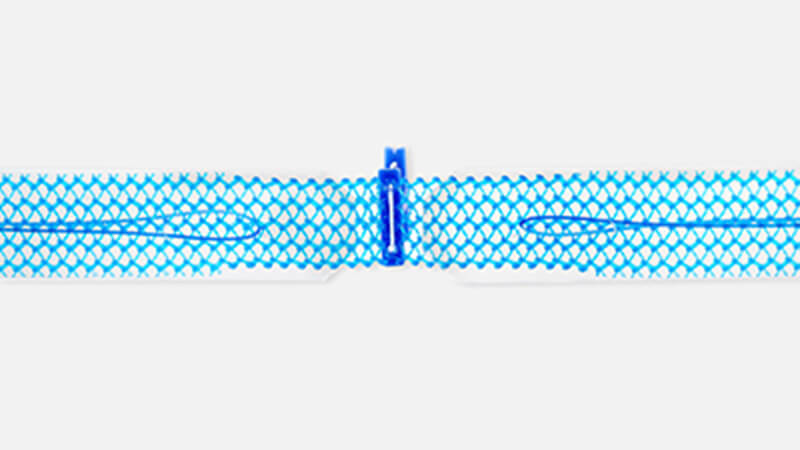
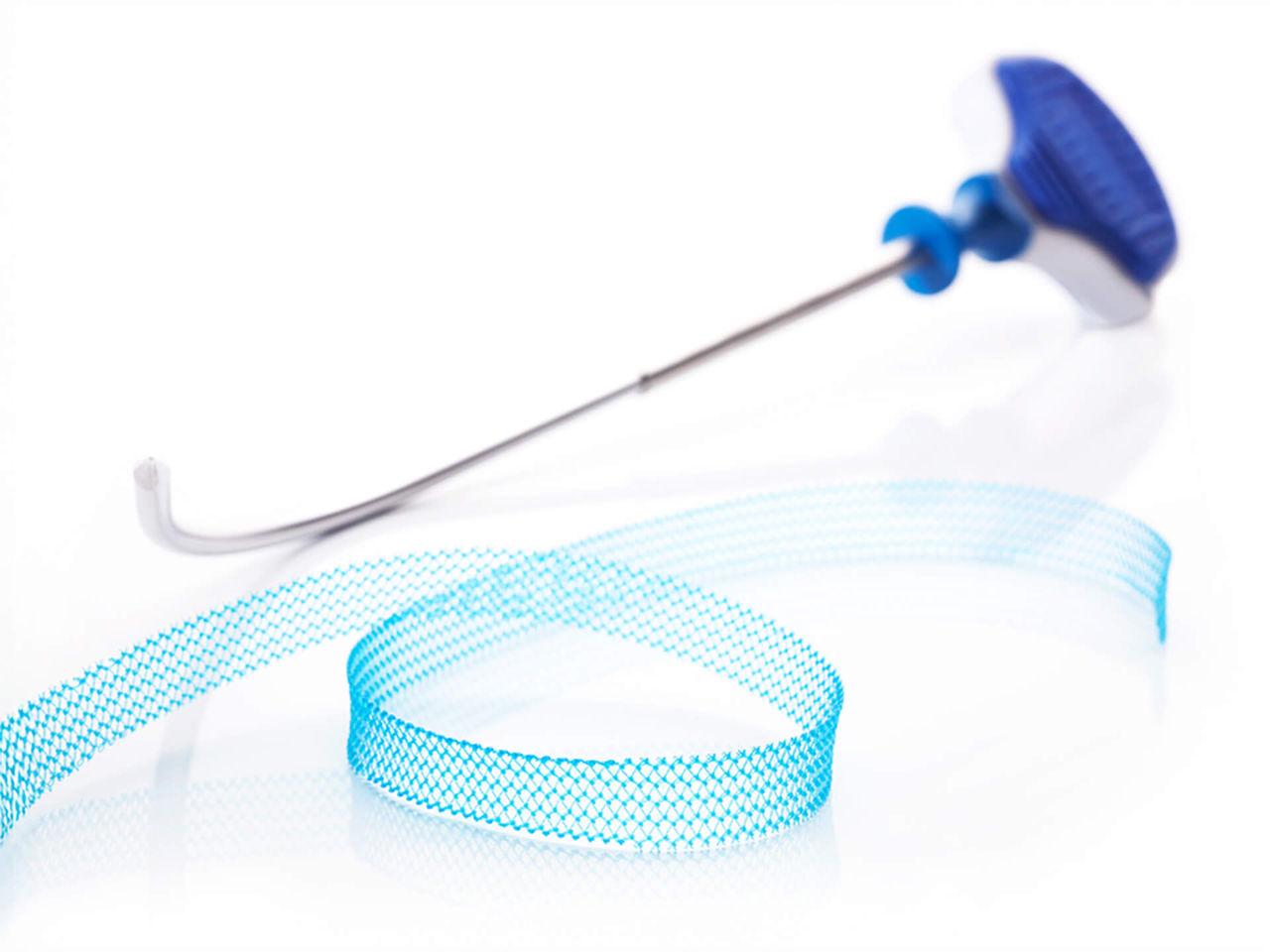
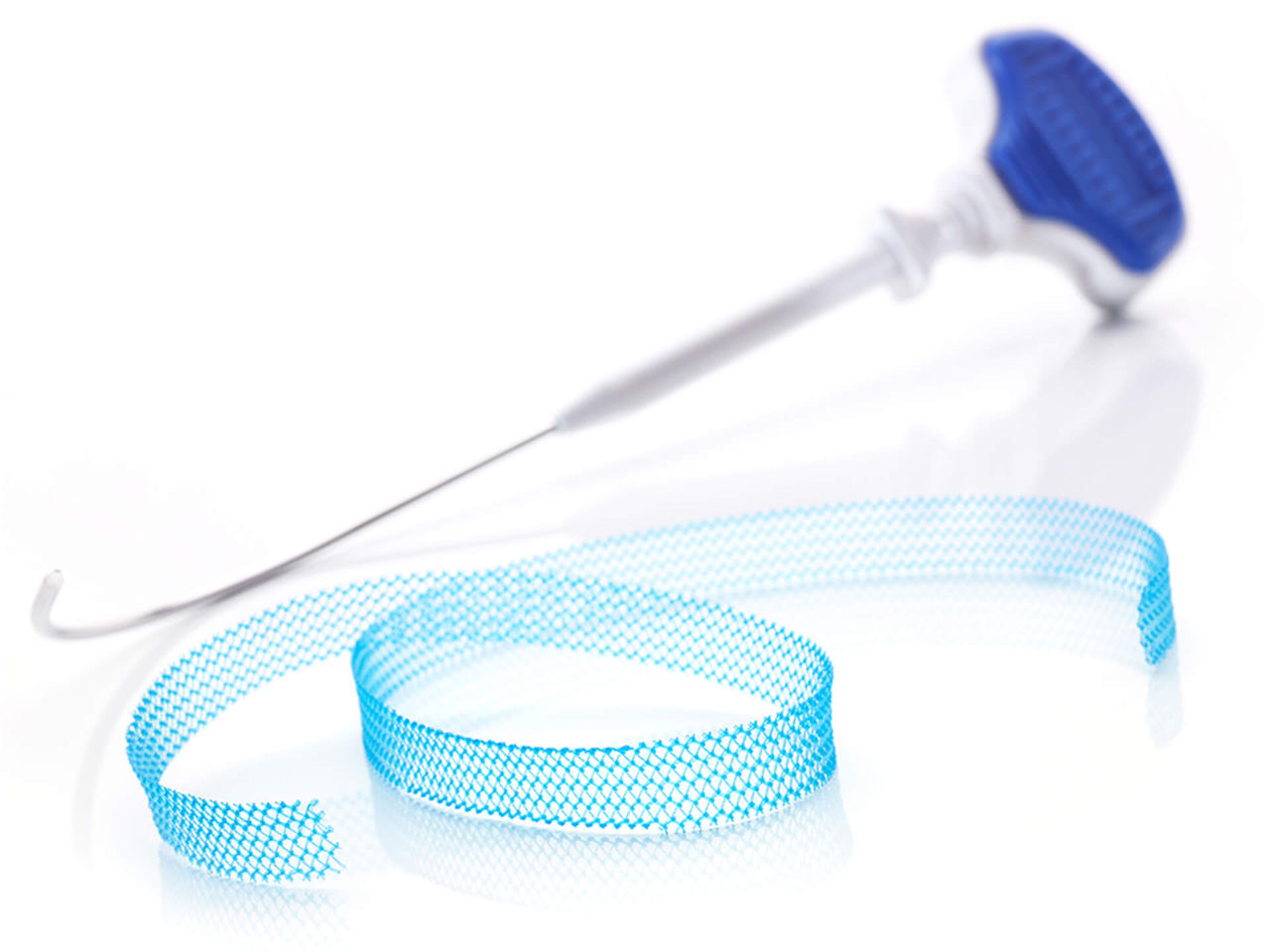
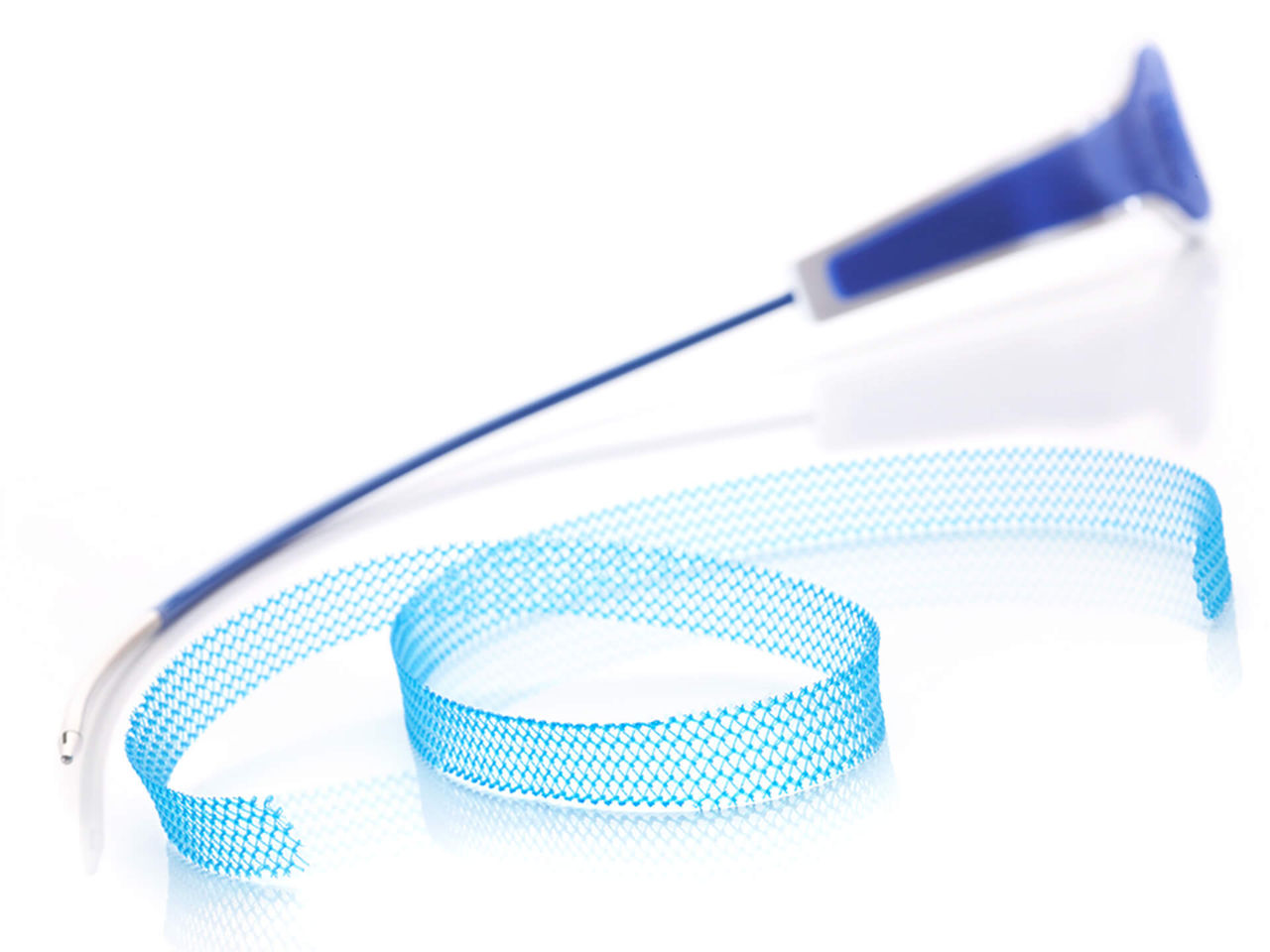
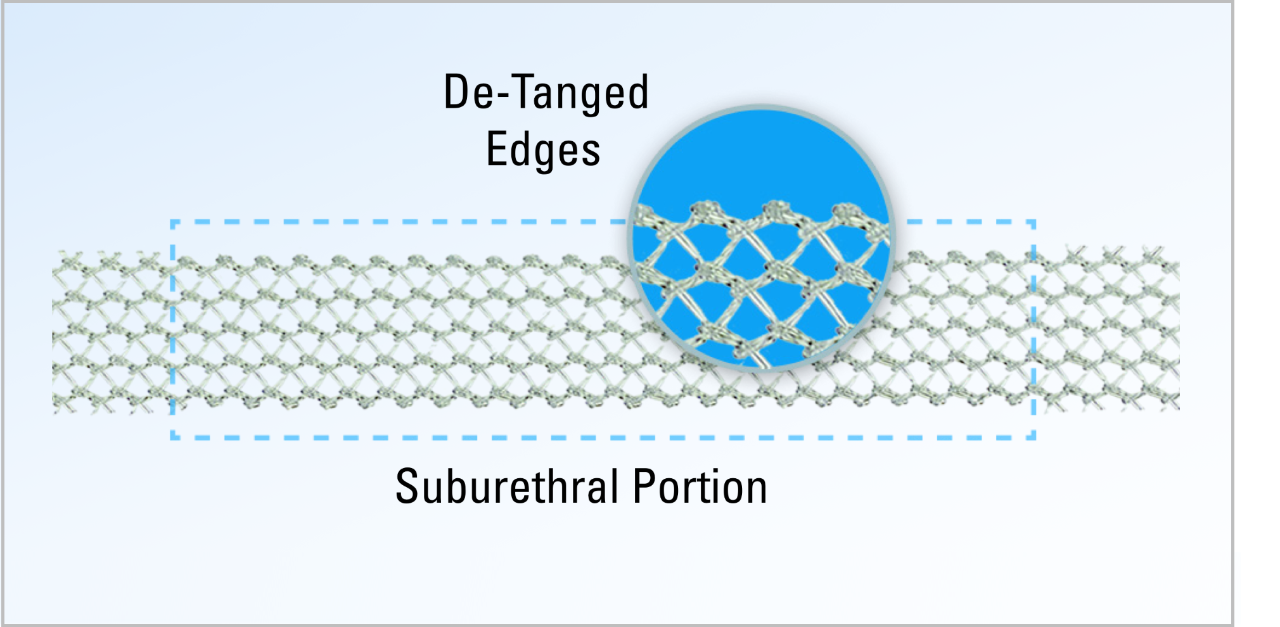
The Ultra sling family is designed to improve provider experience through more precise sling placement. Paired with Boston Scientific’s clinically supported Advantage optical blue mesh, its features are designed to help drive procedural efficiency, mesh visualization, and tensioning consistency.
How it works
Michael Ehlert, M.D., FPMRS, from Minnesota Urology, describes how the design of the new Ultra Retropubic Mid-Urethral Sling is intuitive and physician friendly.
Why choose the Ultra sling family
You asked. We answered.
Physician-driven, patient-centered innovation
Discover the Ultra sling family
Subscribe for updates
Receive emails about the latest female pelvic medicine advances, clinical data, and product news.
Clinical highlights
June 2017, Chevrot et al., published in International Urogynecology Journal
September 2010, Lim et al., published in International Urogynecology Journal
May 2008, Moali et al., published in International Urogynecology Journal Pelvic Dysfunction
October 2008, Agarwala, published in UroToday International Journal
September 2008, Noblett et al., published in International Urogynecology Journal Pelvic Dysfunction
Technical specifications
| Trusted polypropylene mesh2 | |
| Thickness | 0.66 mm |
| Pore size | 1182 μm |
| Fiber size (diameter) | 0.15 mm |
| Weight | 100 g/m2 |
| Mesh length | 44.5 cm |
| Exposed mesh length | ~1 cm |
| Delivery | |
| Sleeve length | 24.7 cm per sleeve |
| Advantage Ultra needle diameter | 5 mm |
| Advantage Fit™ Ultra needle diameter | 2.7 mm |
| Lynx™ Ultra needle diameter | 3.175 mm |
Education and training for urology

Duration: 00:12:18
Date: Jan 2021

Duration: 00:04:39
Date: Jan 2021
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Ultra sling family
Physician-driven innovation
Hear from Michael Ehlert, MD, FPMRS, of Minnesota Urology, regarding his experience with Boston Scientific mid-urethral slings, specifically Ultra.
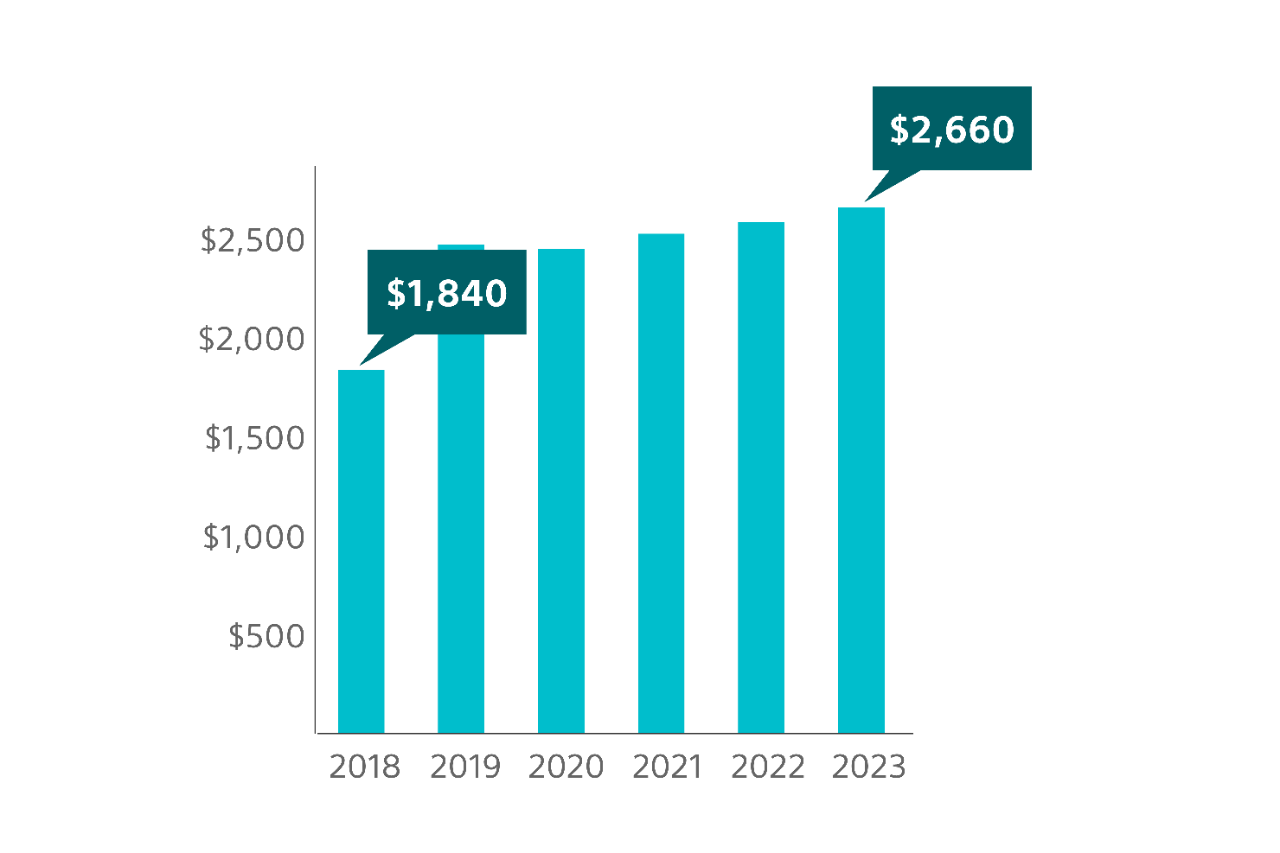
Potential economic impact to institutions
Medicare national average reimbursement for mid-urethral sling surgery, to treat SUI, has seen an overall increase from 2018 to 2023 in both the hospital outpatient department3 and in the ambulatory surgical center (ASC)4
12.7% overall increase
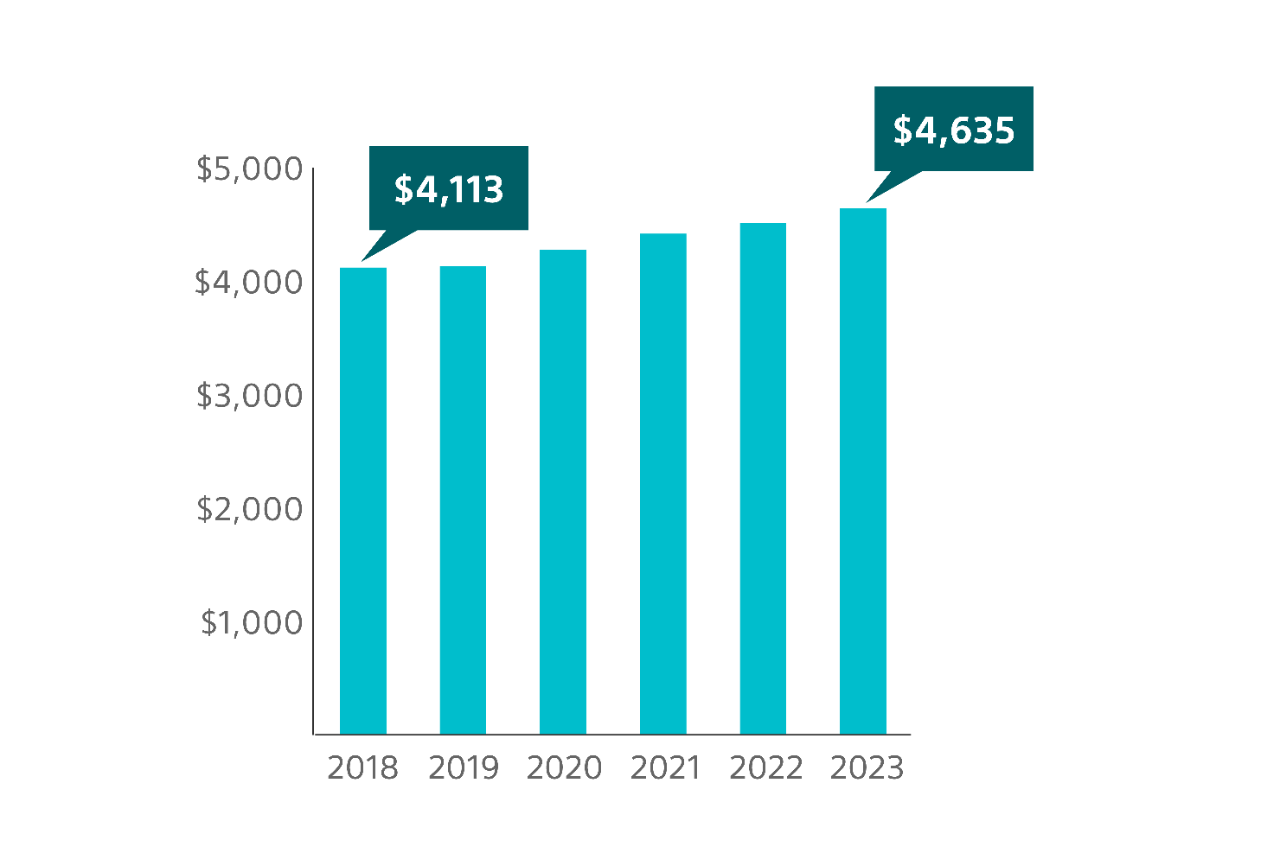
44.6% overall increase
Ordering information
UPN | Description | Quantity |
| Transvaginal approach | ||
M0068502060 | Advantage Ultra Transvaginal Mid-Urethral Sling System | 1 delivery device and 1 mesh assembly |
M0068502160 | Advantage Fit™ Ultra Transvaginal Mid-Urethral Sling System | 1 delivery device and 1 mesh assembly |
| Suprapubic approach | ||
| M0068503060 | Lynx™ Ultra Suprapubic Mid-Urethral Sling System | 2 delivery devices and 1 mesh assembly |
References
- Data on file with Boston Scientific.
- Moalli PA, Papas N, Menefee S, Albo M, Meyn L, Abramowitch SD. Tensile properties of five commonly used mid-urethral slings relative to the TVT. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:655–663.
- Centers for Medicare Medicaid Services. Hospital Outpatient PPS. https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-outpatient. Accessed July 2025.
- Centers for Medicare Medicaid Services. Ambulatory Surgical Center (ASC) Payment. https://www.cms.gov/medicare/payment/prospective-payment-systems/ambulatory-surgical-center-asc. Accessed July 2025.
Caution: U.S. federal law restricts this device to sale by or on the order of a physician trained in use of surgical mesh for repair of stress urinary incontinence. Refer to the instructions for use for this product for complete indications for use, contraindications, warnings, precautions, adverse events, and instructions prior to using this product.
The following adverse events have been reported due to suburethral sling placement, any of which may be ongoing, but are not limited to: as with all implants, local irritation at the wound site and/or a foreign body response may occur, foreign body reaction may be acute or chronic, pain (pelvic, vaginal, groin/thigh, suprapubic, dyspareunia) (acute or chronic), dyspareunia, tissue responses to the mesh implant could include: erosion into organs (urethra, bladder or other surrounding tissues); exposure/extrusion into the vagina, mesh contact with urine via erosion/exposure/extrusion may result in stone formation, scarring/scar contracture, necrosis, fistula formation (acute or chronic), inflammation (acute or chronic), mesh contracture, tissue contracture, vaginal shortening or stenosis that may result in dyspareunia and/or sexual dysfunction, pain with intercourse that may not resolve, exposed mesh may cause pain or discomfort to the patient’s partner during intercourse, sexual dysfunction, including the inability to have intercourse. Like all foreign bodies, the mesh may potentiate an existing infection. Allergic reaction has been reported. Known risks of surgical procedures for the treatment of incontinence include: pain, ongoing pain (pelvic, vaginal, groin/thigh, suprapubic, dyspareunia), severe, chronic pain, apareunia, leg weakness, infection, de novo detrusor instability, complete failure of the procedure/failure to resolve a patient’s stress urinary incontinence, voiding dysfunction (incontinence, temporary or permanent lower urinary tract obstruction, difficulty urinating, pain with urination, overactive bladder, and retention), bruising, bleeding (vaginal, hematoma formation), abscess, vaginal discharge, dehiscence of vaginal incision, edema and erythema at the wound site, perforation or laceration of vessels, nerves, bladder, urethra or bowel may occur during placement. The following additional adverse events have been reported for the Solyx SIS System: dysuria, hematuria. The occurrence of these events may require surgical intervention and possible removal of the entire mesh. In some instances, these events may persist as a permanent condition after surgical intervention or other treatment. Removal of mesh or correction of mesh-related complications may involve multiple surgeries. Complete removal of mesh may not be possible and additional surgeries may not always fully correct the complications.