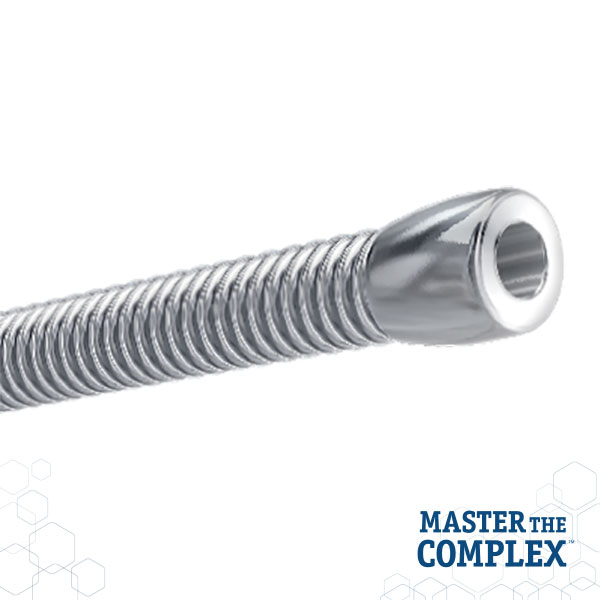
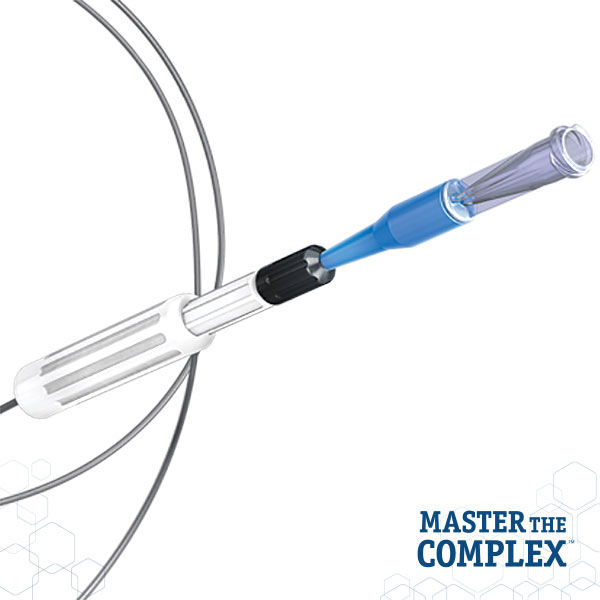
CROSSBOSS™
Coronary CTO Crossing Catheter
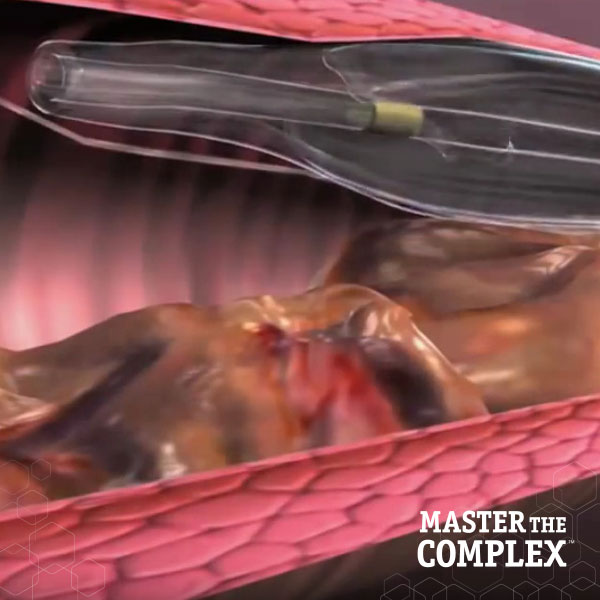
Designed to quickly and safely deliver a guidewire via the true lumen or subintimal pathways, CROSSBOSS Catheter gives you total access to coronary chronic total occlusions.
Key Resources
Explore
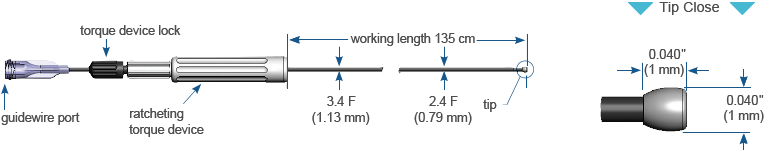
Specifications
Ordering Information
| UPN # | Catalog Number | Product Name/Description | C Code |
|---|---|---|---|
| H749M2000A0 | M-2000-A | CROSSBOSS Catheter | C1725 |
| H749393120SR0 | 39312-0SR | STINGRAY LP Device | C1725 |
| H749M3004A0 | M-3004-A | STINGRAY Guidewire 300 cm | C1769 |
| H749M3010A0 | M-3010-A | STINGRAY Extension Wire | C1769 |
| H749M3012A0 | M-3012-A | STINGRAY Guidewire 185 cm | C1769 |
Clinical Information
FAST-CTO Trial
Facilitated Antegrade Steering Technique in Chronic Total Occlusions
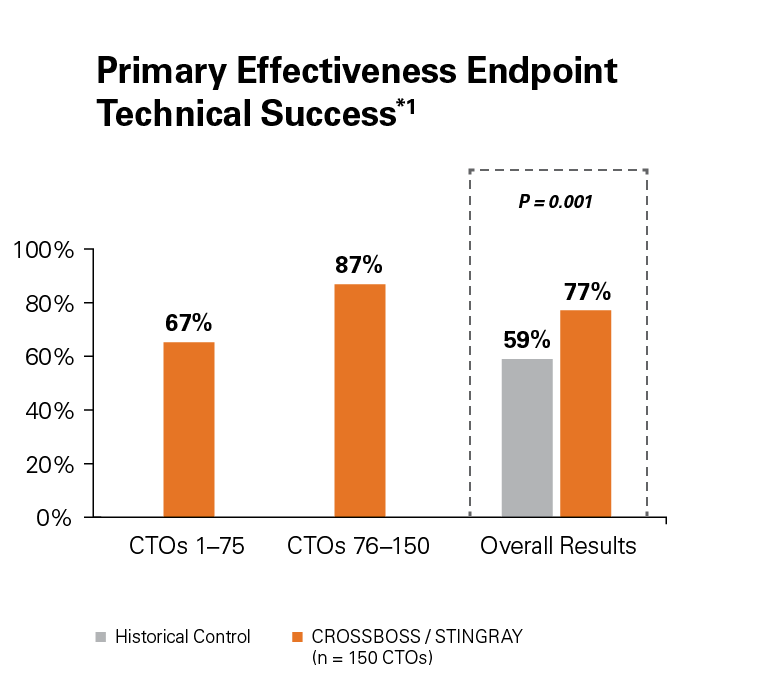
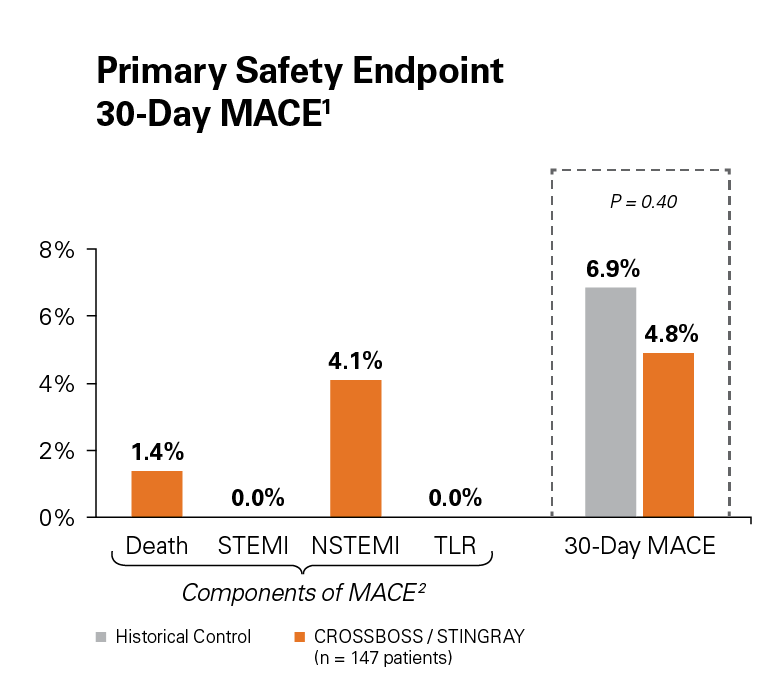
The FAST-CTO trial demonstrated the safety and efficacy of the CROSSBOSS and STINGRAY Coronary CTO Crossing and Re-Entry devices to recanalize coronary CTOs in comparison to historical controls.
- Multi-center, non-randomized US IDE study
- 147 patients in 16 centers
Safe and Effective
Use of the CROSSBOSS and STINGRAY Crossing and Re-Entry devices result in a high technical success, 77%, without increasing complications.1
Reduced Procedure Time
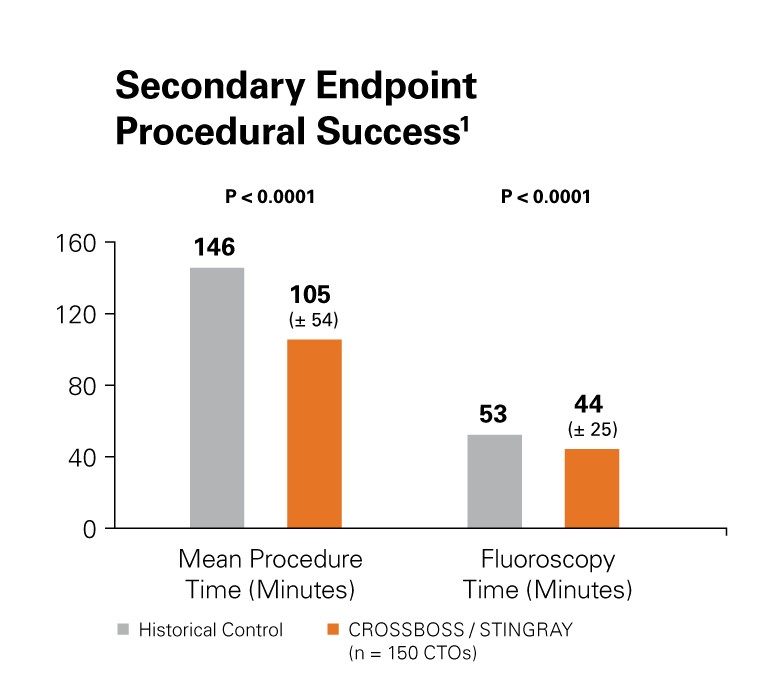
Procedural success was improved with a 28.1% reduction in mean procedure time (minutes) as well as a reduction in fluoroscopy time.1
Principal Investigators
Patrick Whitlow MD, William Lombardi MD, Michael Wyman MD, Craig Thompson MD
Cleveland Clinic, PeaceHealth Medical Center/St Joseph Hospital, Torrance Memorial Medical Center, Yale University School of Medicine