FARAPULSE™
Pulsed Field Ablation System

ADVENT Pivotal Trial
ADVENT was a randomized clinical trial that directly compared FARAPULSE™ PFA to standard-of-care thermal ablation devices (force-sensing radiofrequency ablation —RFA—or cryoballoon ablation—CBA) for the treatment of paroxysmal atrial fibrillation (AF). It was a multi-center, prospective, non-inferiority clinical trial with 1:1 randomization of PFA to thermal ablation evaluating single-procedure, off-drug study endpoints, including:1,2
- Primary Safety
- Primary Effectiveness
- Procedural Characteristics
The most rigorous PFA clinical trial to date:
- Patients randomized to PFA or thermal ablation (RFA or CBA)
- Re-ablations not allowed in 90-day blanking period
- Freedom from Class I/III anti-arrhythmic drug (AAD) after the 90-day blanking period (amiodarone was not allowed at any time)
- Stringent monitoring with 72-hour Holters
- Largest PFA trial with 305 patients treated with PFA
Trial results were collected from 65 thermal operators in 30 centers with 607 randomized patients treated with PFA or thermal ablation and included in the primary results.
The randomized ADVENT trial achieved non-inferiority* in the primary safety and efficacy endpoints when comparing the FARAPULSE PFA System against thermal ablation modalities.
|
Safety outcomes
In the ADVENT trial, the primary safety endpoints were defined as a composite of serious adverse events (SAEs) related to either the use of the ablation catheter or ablation procedure itself, within seven days of the initial procedure and monitored out to 12 months.
The ADVENT clinical trial met the primary safety endpoint for non-inferiority of FARAPULSE PFA to thermal ablation (posterior probability >0.999). In the secondary safety endpoint, FARAPULSE demonstrated superiority to standard-of-care thermal ablation by having significantly less PV cross-sectional narrowing at three months. These results continue to support that FARAPULSE PFA energy is cell selective with no reported thermal complications (persistent phrenic nerve palsy, esophageal fistula, or pulmonary vein stenosis).
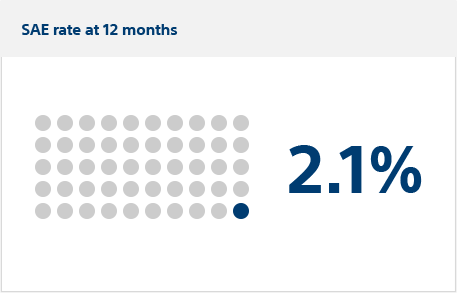
Primary safety endpoint:
Severe adverse events (SAEs)
Serious adverse events occurred in six FARAPULSE PFA patients (estimated incidence: 2.1%) vs. four thermal patients (1.5%), meeting the criterion for non-inferiority (posterior probability >0.999).
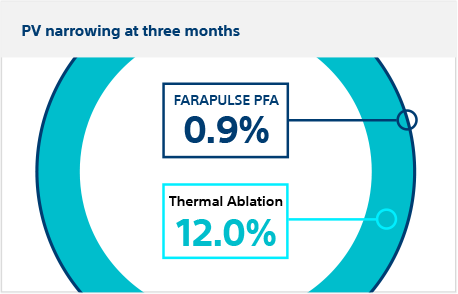
Secondary safety endpoint:
Pulmonary vein narrowing
Patients returned at three months for CT or MRI scan. There was significantly less pulmonary vein cross-sectional narrowing in FARAPULSE PFA patients (0.9%) vs thermal ablation patients (12.0%), meeting the criterion for superiority (posterior probability >0.999)
ADVENT is the first and only randomized clinical trial in which PFA has been shown to be non-inferior* to standard-of-care thermal ablation (RFA and CBA) for primary safety endpoints.
|
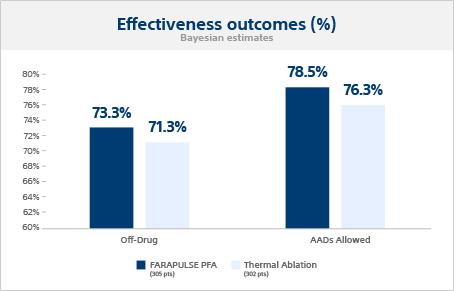
Effectiveness outcomes
Primary effectiveness outcome
The Bayesian estimated single-procedure, off-drug treatment success probabilities were 73.3% for FARAPULSE and 71.3% for thermal ablation, meeting the criterion for non-inferiority.
Additional effectiveness outcome
When anti-arrhythmic drugs (AADs) were allowed post-blanking, the 12-month success probabilities were 78.5% for FARAPULSE and 76.3% for thermal ablation.
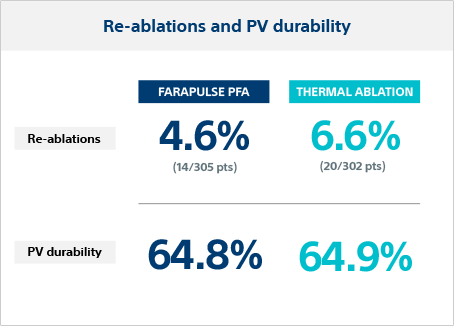
PV durability in re-ablation
Repeat ablation was performed in 4.6% of FARAPULSE patients and 6.6% in thermal ablation patients. The PV durability in re-ablated patients was 64.8% per vein (28.6% per patient) for FARAPULSE and 64.9% per vein (26.3% per patient) for thermal.
The ADVENT FARAPULSE 12-month effectiveness outcome was non-inferior* to thermal ablation devices, although operators had limited experience with the PFA system and were considered expert thermal operators.
|
Procedural characteristics
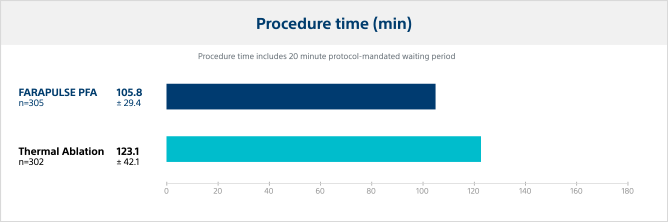
Procedure time
In the ADVENT trial, PFA with FARAPULSE demonstrated reduced procedure times by 14% versus thermal ablation modalities.
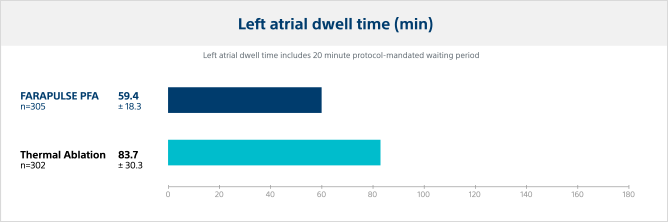
Left atrial (LA) dwell time
Pulsed field ablation catheter LA dwell time was significantly lower, reducing the LA dwell time by 29% versus thermal ablation modalities.
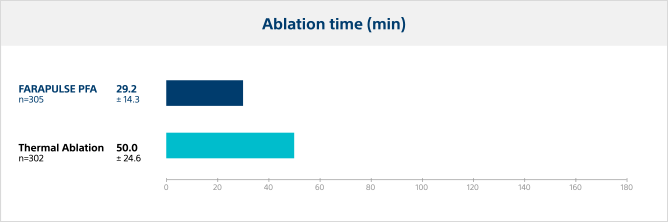
Ablation time
The time from first ablation to last ablation was significantly lower for PFA, with an average reduction in ablation time of 42% compared to thermal ablation modalities.
Fluoroscopy time
Pulsed field ablation required a longer duration of fluoroscopy versus thermal ablation, as expected with operators who are new to the PFA system.
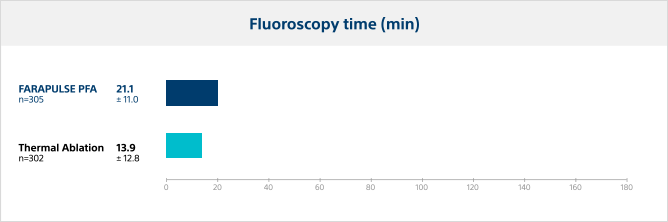
In the ADVENT Pivotal Trial, FARAPULSE PFA procedure times were significantly shorter with less variability than thermal ablation.
|

ADVENT study overview3

Blanking period (90 days)
- Re-ablations: only cavotricuspid isthmus ablation (CTI) ablations allowed, as needed
- AADs: allowed, as needed (Amiodarone was not allowed at any point)
Follow-up (at 12 months post-op)
- Re-ablations: none allowed
- AADs: use of Class I/III AADs not allowed after blanking
Study enrollment3
An initial sample size of 706 patients was used (626 randomized; 80 roll-in), leading to a patient modified Intent-to-Treat (mITT) cohort of 607 for primary results. mITT subjects received energy delivery for PVI with the randomized endocardial ablation catheter at an Index/Rescheduled Index Procedure.
Patients receiving treatment were divided across non-thermal (n=305) and thermal (302 total; RFA n=167, CBA n=135) arms.
Safety
Primary safety endpoint was a composite of pre-defined SAEs that were related to the use of the ablation catheter or ablation procedure occurring within 7 days of the index procedure. PV stenosis and atrio-esophageal fistula events were monitored to 12 months.
A secondary safety endpoint measured pulmonary vein (PV) narrowing and tested for superiority of PFA to thermal ablation. Changes in PV dimensions were measured at baseline and 90 days to characterize differences in PV remodeling and stenosis between PFA and thermal ablation.
Additional outcomes
- Silent cardiac event (SCE) and silent cerebral lesion (SCL) rates
- Procedure durations
- Quality of life
- Learning curve effects
- Rate of re-ablation and PVI durability at re-ablation
- Early recurrence of AF
- Post-blanking cardioversions
- Arrhythmia hospitalizations

In the ADVENT Pivotal Trial, FARAPULSE demonstrated:
- Non-inferiority for both the primary safety and effectiveness outcomes compared to thermal technology.*
- Significantly less pulmonary vein cross-sectional narrowing compared to thermal ablation.*
- Significantly shorter procedure times, reduced LA dwell time and total ablation time versus thermal ablation. Lower standard deviations across these characteristics also indicate less variability within the PFA procedures.
* posterior probability >0.999

References:
1. Reddy et al. Pulsed field ablation or conventional thermal ablation for paroxysmal atrial fibrillation. Presented at: ESC 2023; August 27, 2023, Amsterdam, NL.
2. Reddy, et al., Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. New England Journal of Medicine (2023). In press.
3. Reddy V, Lehmann JW, Gerstenfeld EP, et al. A randomized controlled trial of pulsed field ablation versus standard-of-care ablation for paroxysmal atrial fibrillation: The ADVENT trial rationale and design. Heart Rhythm O2. 2023 Mar 08;4(5):317-28. doi: 10.1016/j.hroo.2023.03.001
