Boston Scientific accounts are for healthcare professionals only.

AMS 800™ Artificial Urinary Sphincter
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Training
- Resources
- Ordering information
The gold standard
More than 250,000 AMS* artificial urinary sphincters have been sold since the technology was initially developed in 1972. Today the AMS 800 Artificial Urinary Sphincter is the gold standard treatment for moderate to severe male stress urinary incontinence (SUI) following prostate surgery.
How it works
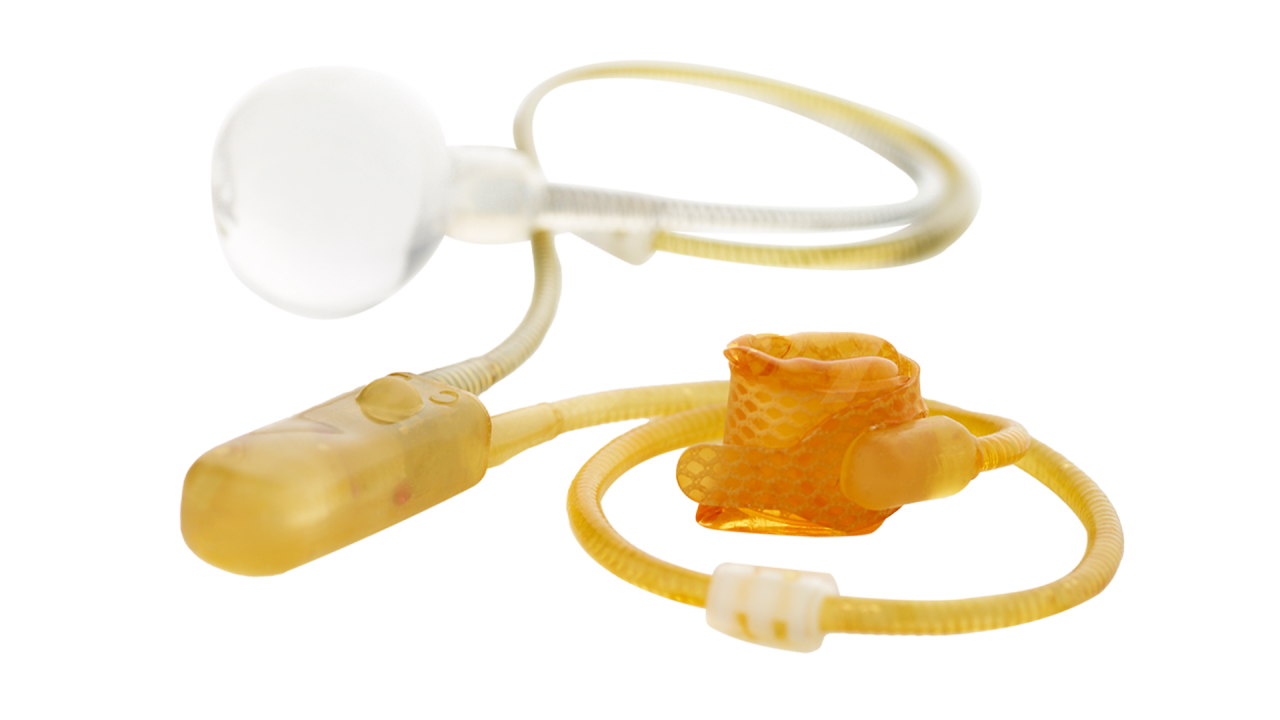
The AMS 800 Artificial Urinary Sphincter is a completely concealed, implantable, fluid-filled, solid silicone elastomer device that treats male SUI resulting from intrinsic sphincter deficiency following prostate surgery. It mimics normal sphincter function by opening and closing the urethra at the control of the patient.
Why choose the AMS 800
Patient satisfaction
Studies have shown:
- 94% of 148 patients surveyed would “definitely” or “probably” recommend the surgery to a family member or friend.1
- More than 90% of 278 patients surveyed reported they would have the surgery again.2
- Out of 105 patients implanted with an AMS 800 AUS, 90% of patients with a history of urethroplasty (n=30) and 94.7% of non-urethroplasty patients (n=75) were satisfied with the overall surgical outcome.3
*Includes all versions of AMS (now Boston Scientific) artificial urinary sphincters.
References:
Linder BJ, Rivera ME, Ziegelmann MJ, et al. Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic. Urology. 2015 Sep;86(3):602-7.
Viers BR, Linder BJ, Rivera ME, et al. Long-Term Quality of Life and Functional Outcomes among Primary and Secondary Artificial Urinary Sphincter Implantations in Men with Stress Urinary Incontinence. J Urol. 2016 Sep;196(3):838-43.
Sayedahmed K, Olianas R, Kaftan B, et al. Impact of previous urethroplasty on the outcome after artificial urinary sphincter implantation: a prospective evaluation. World J Urol. 2020 Jan;38(1):183-191.
Clinical publications
Sep. 2016
Apr. 2016
Apr. 2013
Pressure-regulating balloon
| Non-InhibiZone™ Antibiotic Surface Treatment |
| 51-60cm H20 |
| 61-70cm H20 |
| 71-80cm H20 |
Occlusive cuff
| With InhibiZone™ Antibiotic Surface Treatment | Non-InhibiZone™ Antibiotic Surface Treatment |
| 3.5cm | 3.5cm |
| 4.0cm | 4.0cm |
| 4.5cm | 4.5cm |
| 5.0cm | 5.0cm |
| 5.5cm | 5.5cm |
| 6.0cm | 6.0cm |
| 6.5cm | 6.5cm |
| 7.0cm | 7.0cm |
| 7.5cm | 7.5cm |
| 8.0cm | 8.0cm |
| 9.0cm | 9.0cm |
| 10.0cm | 10.0cm |
| 11.0cm | 11.0cm |
EDUCARE registration
Online medical training and education courses
The EDUCARE online platform makes medical education and training relevant, comprehensive, personal, and accessible. Register to access procedural videos, case studies, and training resources.
These healthcare professional resources are provided for use by practicing physicians and allied healthcare professionals. This download center is not intended for direct use by patients or consumers.
Resource Downloads
Product wordmarks
Product photography
Templated Word documents and banner ads
Patient education
Patient testimonials
For more patient education, visit Fix Incontinence.
Patient downloads
- Fix Your Incontinence - Patient Education Brochure
- Life After Prostate Cancer Book
- Life After Prostate Cancer Book - Spanish
- Prostate Cancer Brochure
- Prostate Cancer Brochure - Spanish
- Prostate Cancer Survivorship Questionnaire with SHIM
- Male Continence Treatment Options Brochure
- Male Continence Treatment Options Brochure - Spanish
- SUI Patient Brochure
For more patient education, visit Fix Incontinence.
AMS 800™ Artificial Urinary Sphincter
| Control Pump With InhibiZone™ (IZ) Antibiotic Surface Treatment | ||
|---|---|---|
| Name | Order number | GTIN number |
| Control Pump – InhibiZone | 72404127 | 00878953003078 |
| Control Pump, Non-InhibiZone (IZ) Antibiotic Surface Treatment | ||
|---|---|---|
| Name | Order number | GTIN number |
| Control Pump | 72400098 | 00878953000688 |
| Pressure Regulating Balloon | ||
|---|---|---|
| Name | Order number | GTIN number |
| 51-60 cm H2O | 72400023 | 00878953000619 |
| 61-70 cm H2O | 72400024 | 00878953000626 |
| 71-80 cm H2O | 72400025 | 00878953000633 |
| Occlusive Cuff With InhibiZone (IZ) Antibiotic Surface Treatment | ||
|---|---|---|
| Name | Order number | GTIN number |
| 3.5 cm IZ | 720157-01 | 00878953005652 |
| 4.0 cm IZ | 72404130 | 00878953003085 |
| 4.5 cm IZ | 72404131 | 00878953003092 |
| 5.0 cm IZ | 72404132 | 00878953003108 |
| 5.5 cm IZ | 72404133 | 00878953003115 |
| 6.0 cm IZ | 72404134 | 00878953003122 |
| 6.5 cm IZ | 72404135 | 00878953003139 |
| 7.0 cm IZ | 72404136 | 00878953003146 |
| 7.5 cm IZ | 72404137 | 00878953003153 |
| 8.0 cm IZ | 72404138 | 00878953003160 |
| 9.0 cm IZ | 72404140 | 00878953003177 |
| 10.0 cm IZ | 72404142 | 00878953003184 |
| 11.0 cm IZ | 72404144 | 00878953003191 |
| Occlusive Cuff, Non-InhibiZone (IZ) Antibiotic Surface Treatment | ||
|---|---|---|
| Name | Order number | GTIN number |
| 3.5 cm IZ | 720133-01 | 00878953005461 |
| 4.0 cm IZ | 72400160 | 00878953000718 |
| 4.5 cm IZ | 72400161 | 00878953000725 |
| 5.0 cm IZ | 72400162 | 00878953000732 |
| 5.5 cm IZ | 72400163 | 00878953000749 |
| 6.0 cm IZ | 72400164 | 00878953000756 |
| 6.5 cm IZ | 72400165 | 00878953000763 |
| 7.0 cm IZ | 72400166 | 00878953000770 |
| 7.5 cm IZ | 72400167 | 00878953000787 |
| 8.0 cm IZ | 72400168 | 00878953000794 |
| 9.0 cm IZ | 72400170 | 00878953000800 |
| 10.0 cm IZ | 72400172 | 00878953000817 |
| 11.0 cm IZ | 72400174 | 00878953000824 |
| AMS 800™ Accessory Kit | ||
|---|---|---|
| Name | Order number | GTIN number |
| 720066-01 | 00878953004938 |
| Deactivation Package | ||
|---|---|---|
| Name | Order number | GTIN number |
| 72400095 | 00878953000664 |
| Insertion Package | ||
|---|---|---|
| Name | Order number | GTIN number |
| Insertion Package (tubing passers) | 72100005 | 00878953000558 |
| AMS Quick Connect Assembly Tool | ||
|---|---|---|
| Name | Order number | GTIN number |
| AMS Quick Connect Assembly Tool | 72400271 | 00878953000831 |
| SKW Deep Scrotal Retract System | ||
|---|---|---|
| Name | Order number | GTIN number |
| SKW Deep Scrotal Retraction System | 72403867 | 60888937012400 |