Get the most from every pulse
Lower tracking force combined with improved flexibility and kink resistance means you’re in control of delivery.1 Cross challenging anatomies and calcified lesions with confidence.
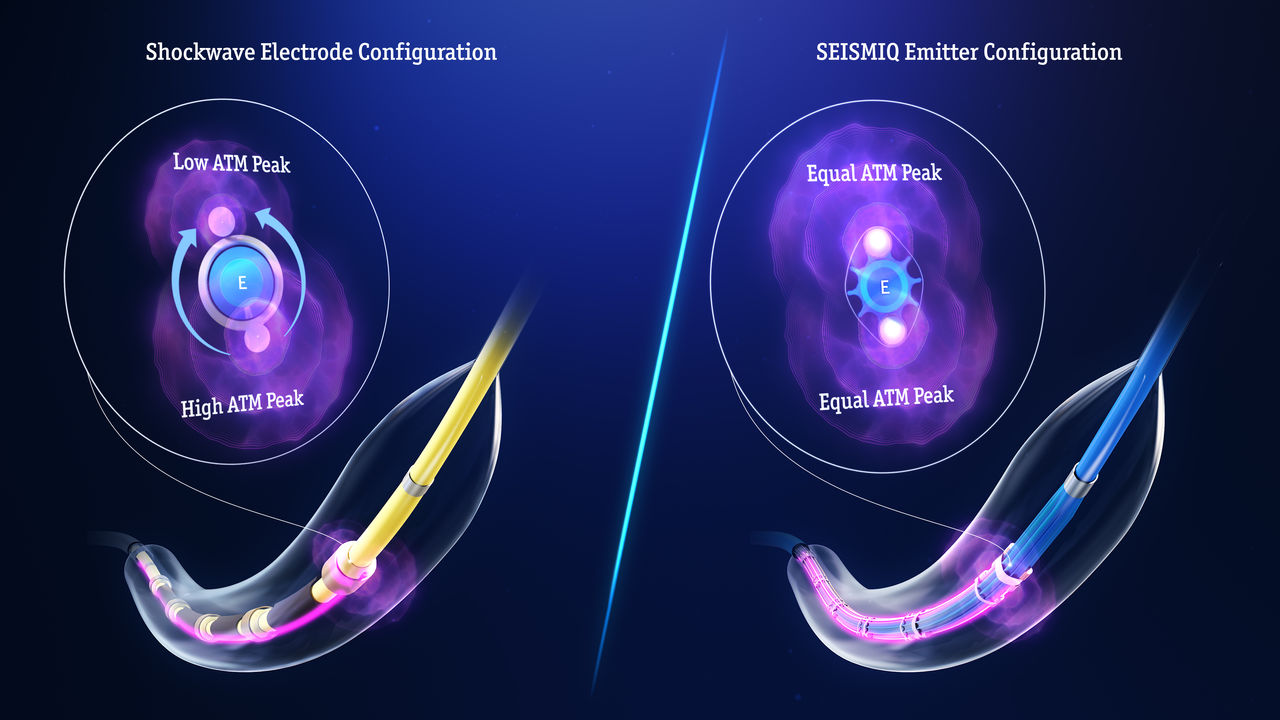
The most consistent pressure per pulse
Consistent acoustic pressure across all 5 emitter stations.
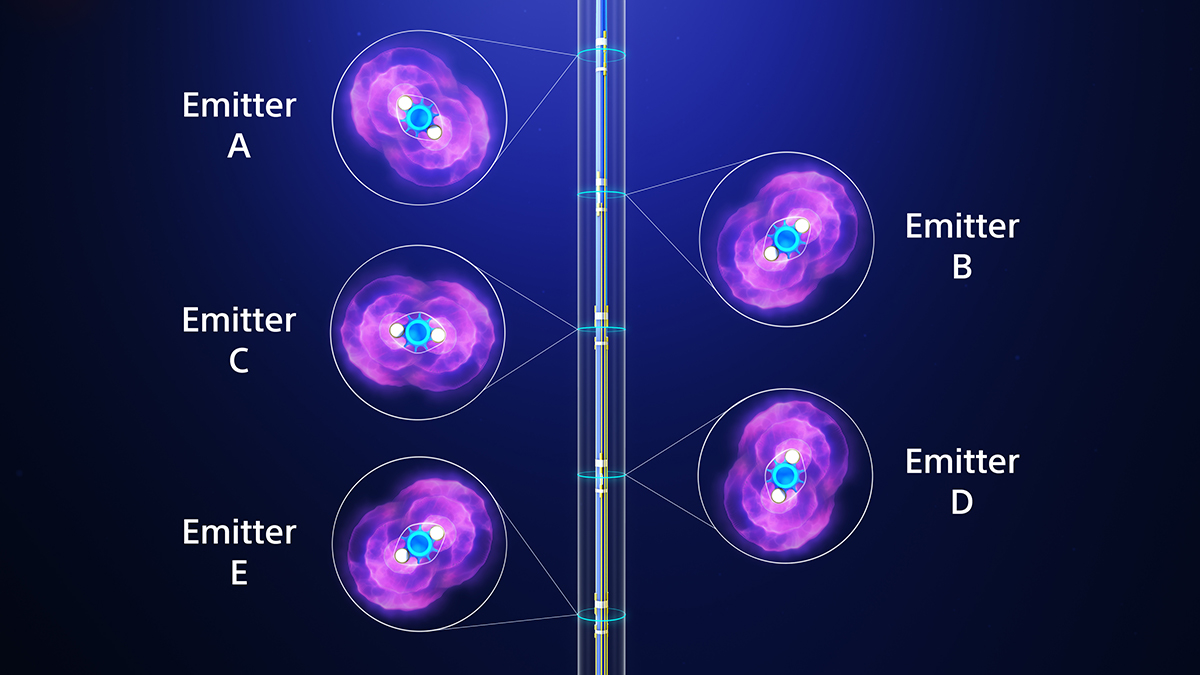
Target eccentric and nodular calcium
360-degree helical emitter rotation for targeted and precise therapy delivery.

Select your pulses
Emitter selectivity gives physicians precise control over where acoustic pressure is delivered inside the vessel, enabling them to preserve pulses and focus therapy exactly where it’s needed.
