Eluvia DES Elutes Paclitaxel When It’s Most Critical. Zilver PTX’s Falls Short.
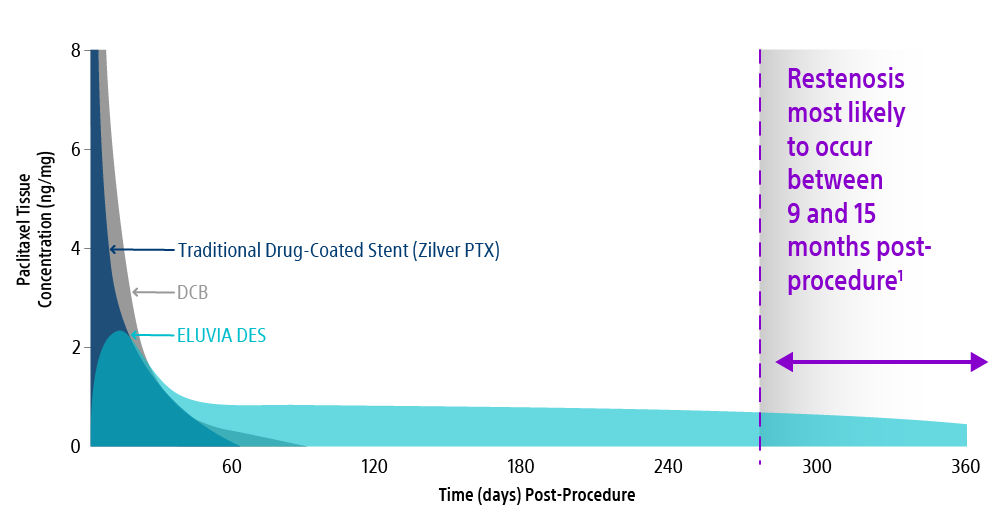
Eluvia DES is the only SFA drug-eluting stent with a polymer coating, enabling a sustained drug release to match the SFA restenotic cascade.2
The polymer:
- Maintains a steady release of paclitaxel during the height of restenosis
- Protects the drug from dissolving in the blood
- Ensures highly controlled drug delivery to the target lesion.
Eluvia DES releases paclitaxel for ~365 days. In comparison - Zilver PTX, a drug-coated stent without a polymer, releases all paclitaxel within 72 hours and paclitaxel is detectable in tissue through only 56 days.3
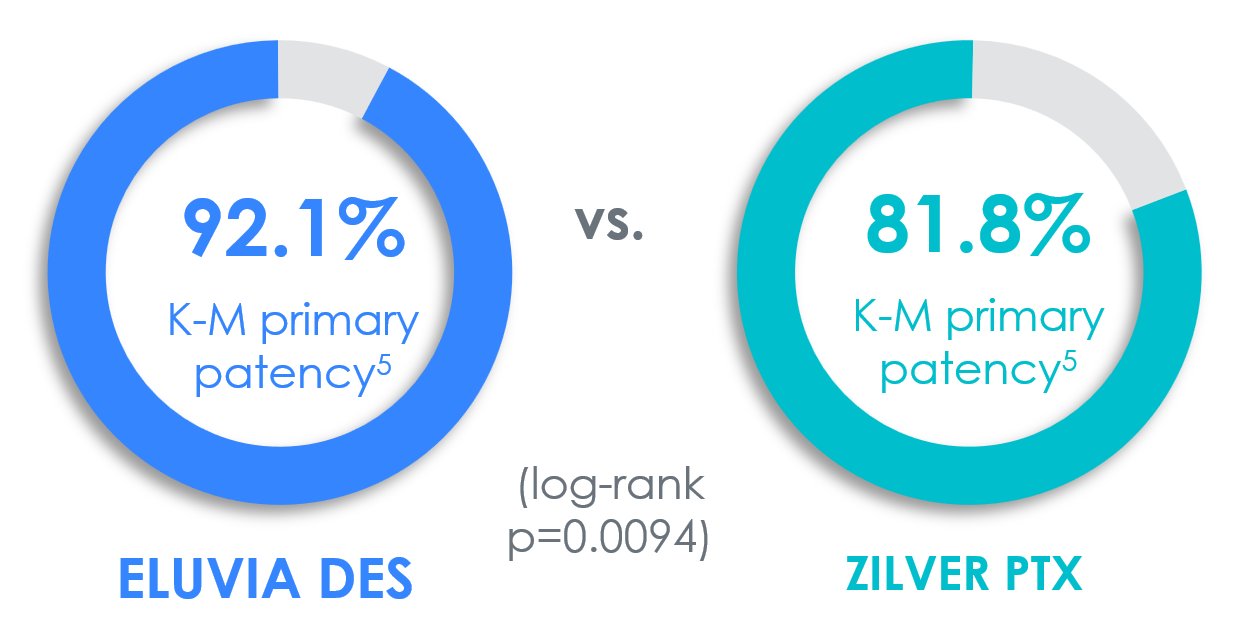
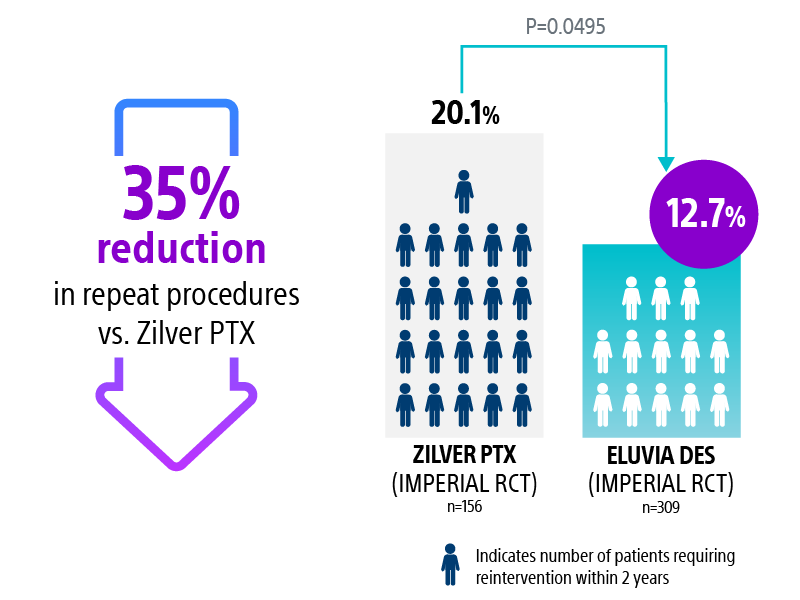
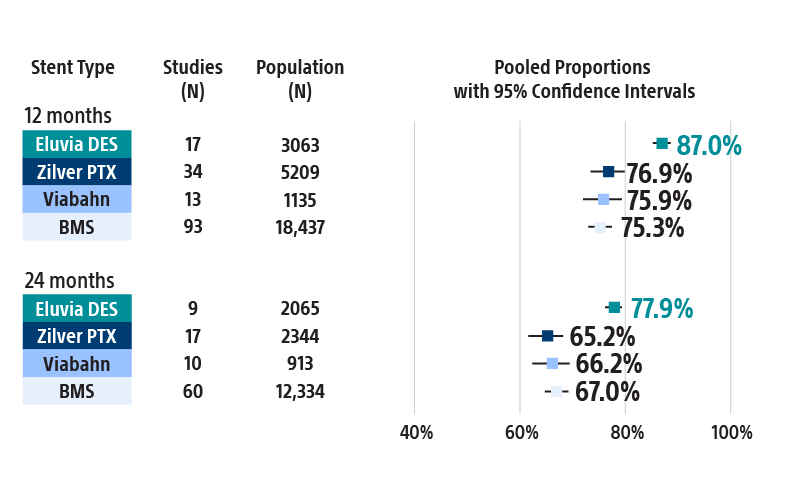
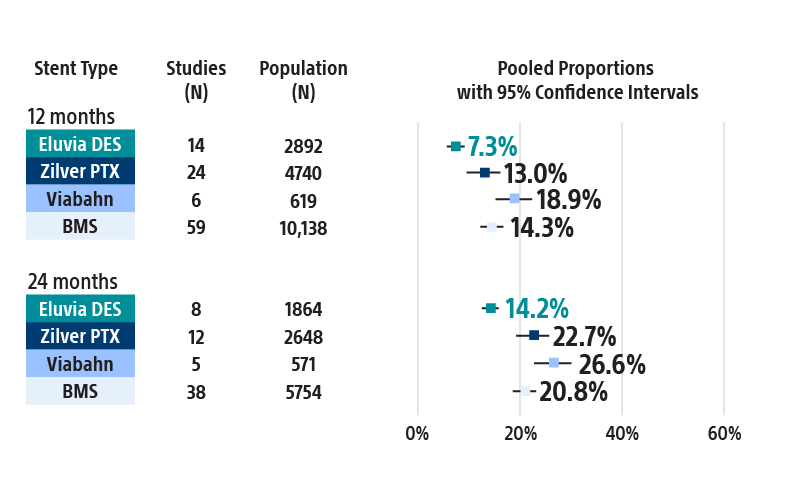
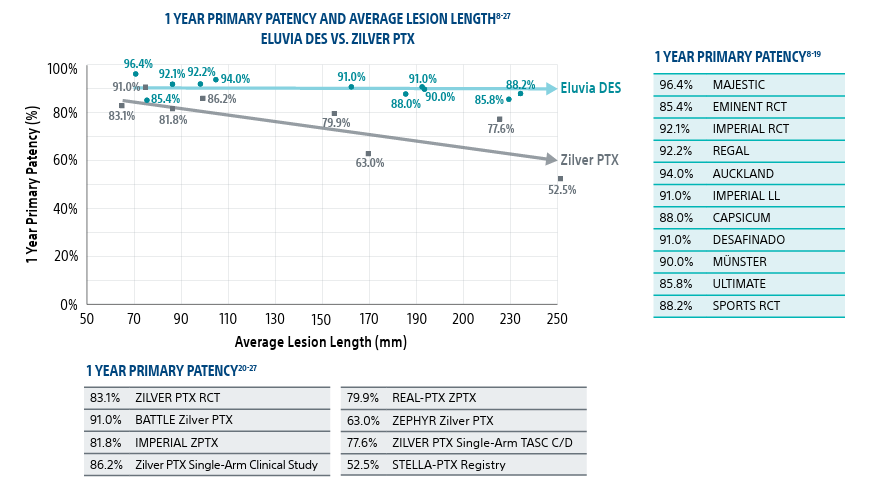
Most restenosis and TLR events occur within the first 12–24 months. Choose the stent that is engineered for efficient and sustained drug elution through the height of restenosis.