Boston Scientific accounts are for healthcare professionals only.
Different Outcomes. Different Technology.
When you’ve done everything right but aren’t getting the results you want for your SCS patient, try something different. Using the first and only MRI conditional adapters, offered exclusively from Boston Scientific, you can connect to leads from other manufacturers while maintaining your patient’s MRI access. Experience the difference today.
Experience proven salvage success
Clinical data shows that the majority of patients studied who did not respond to another manufacturer’s SCS therapy found pain relief when their leads were connected to the Boston Scientific SCS System.1,2
60%
salvage success for failed competitive trials1
60%
salvage success for failed competitive implants2
Long-term salvage success
In a published clinical study, salvage patients reported durable pain relief out to an average of 1.4 years2
At no cost to you, your Boston Scientific representative can come to your clinic during your competitive trial and use MRI conditional adapters to help salvage your patient’s trial.
Different technology that gets it right the first time
Maximize your trials
Boston Scientific’s FAST™ Therapy (Fast-Acting Sub-Perception Therapy) delivers profound, paresthesia-free pain relief in as little as 2 minutes, allowing your patients to experience relief before they even leave the clinic.3-7†
†Disclaimer: These outcomes reflect approximated data as determined in the referenced studies.
A slow 2-day wash-in period wastes
~30%
of a 7-day trial*
Physicians' clinical perspectives
Data-driven swaps deliver relief
60% of patients that swap to Boston Scientific have positive results, and they obtain pain relief and function. So, we did a full system swap (leads and IPG). She has a WaveWriter Alpha and is reporting 75% pain relief, improved function, and she’s quite happy with the therapy.”
– Michael Esposito, MD
FAST Therapy drives trial efficiency
What really makes a difference now is we have patients know within two days, three days if they want to proceed with a permanent. The onset is so quick that it’s really changed completely how I practice.”
– Manny Gage, MD

Ready to see the difference for yourself?
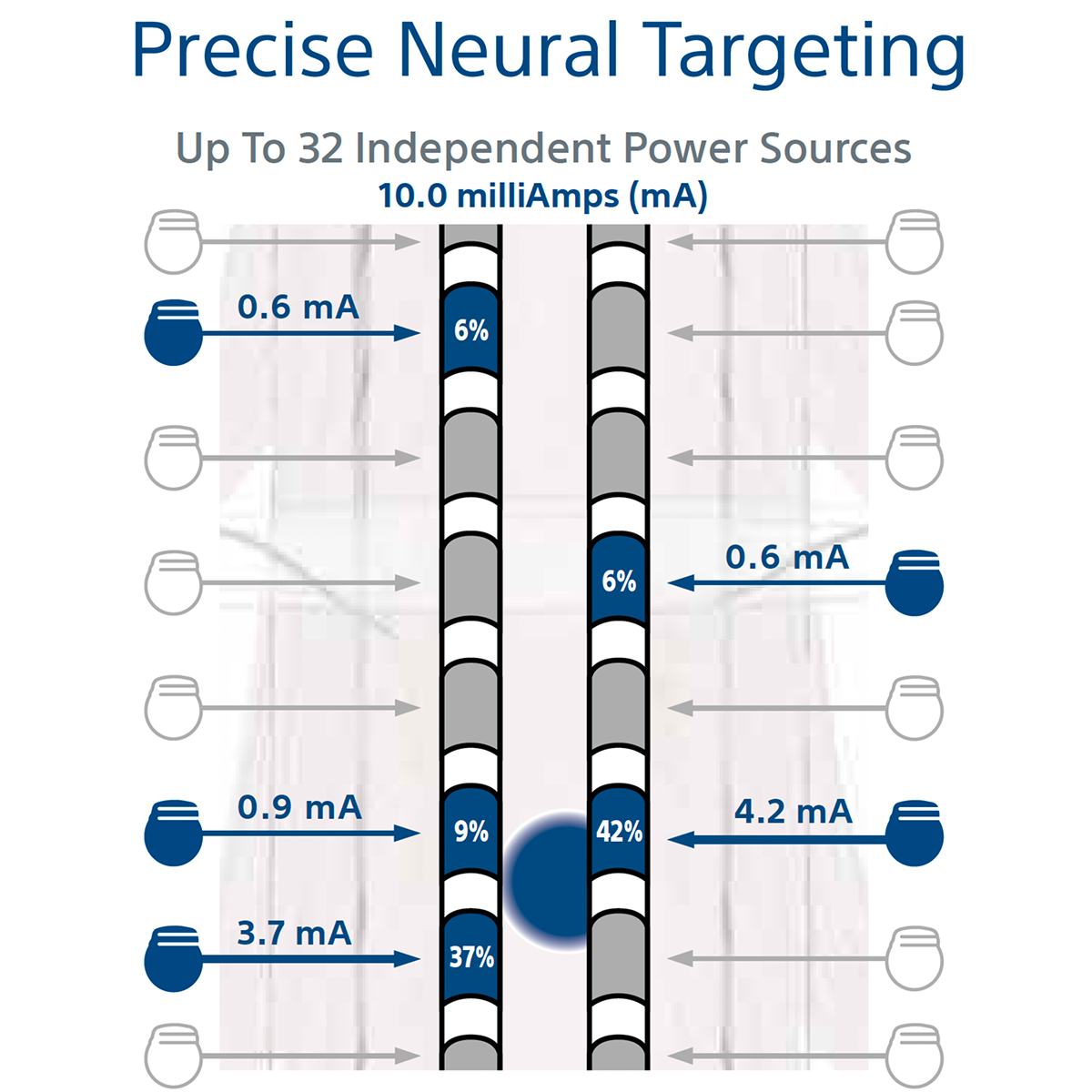
Why is WaveWriter Alpha™ Spinal Cord Stimulator (SCS) System Different?
View Boston Scientific Spinal Cord Stimulator System Indications, Safety, and Warnings
Warning: Stimulation modes. Only paresthesia-based stimulation mode has been evaluated for effectiveness in the diabetic peripheral neuropathy (DPN) population
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.
*2 days is approximately 30% of a 7-day trial per 2 days / 7 days = 0.285 ≈ 30%.
** Refer to https://www.bostonscientific.com/elabeling/us/en/home.html for more information
‡ Refer to the Precision M8 Adapter Directions for Use for indications, warnings and cautions. The Precision M8 Adapter is compatible with the following Medtronic lead and extension model numbers: 39286, 39565, 3778, 3878, 3777, 3877, 3776, 3876, 3874, 3873, 3875, 37081, 37083, 37082, 977A160, 977A175, 977A190, 977A260, 977A275, 977A290, 977D160, 977D260
Refer to the “Precision™ S8 Adapter Directions for Use” for indications, warnings and cautions. The Precision™ S8 Adapter is compatible with the following St Jude Lead, Adapter and Extension model numbers: 3283, 3268, 3269, 3280, 3244, 3262, 3263, 3245, 3264, 3265, 3208, 3210, 3288, 3289, 3214, 3219, 3228, 3183, 3186, 3189, 3191, 3086, 3286, 2283, 3341, 3342, 3343, 3346, 3382, 3383, 3386.
§The Boston Scientific SCS Implantable Pulse Generators, OR Cables, and Lead Extensions can be connected to the following compatible Nevro Leads and Lead Extensions: LEAD2008-25B, LEAD2008-35B, LEAD2008-60B, LEAD1058-30B, LEAD1058-50B, LEAD1058-70B, LEAD1058-90B, TLEAD1058-50B, TLEAD1058-70B, TLEAD1058-90B. Warning: The Nevro Leads and Lead Extensions have not been tested for compatibility with an MRI environment when used with the Boston Scientific SCS Systems.
- Haider N, Ligham D, Quave B, et al. Spinal cord stimulation (SCS) trial outcomes after conversion to a multiple waveform scs system. Neuromodulation. 2018;21(5):504-507.
- Rigoard P, Billot M, Bougeard R, et al. Improved outcomes and therapy longevity after salvage using a novel spinal cord stimulation system for chronic pain: multicenter, observational, European case series. J Clin Med. 2024;13(4):1079
- Gage E., et al. Rapid Onset of Analgesia during Trial Period Utilizing Fast-Acting Sub-Perception Therapy SCS [Abstract] NANS 2023 Annual Meeting, Jan 12-15, 2023.
- Anitescu M., et al. Utilizing SCS Designed to Engage Surround Inhibition Using Fast-Acting Sub-Perception Therapy (FAST): Prospective, Multicenter, Long-Term Outcomes [Abstract] NANS 2025 Annual Meeting, Jan 30-Feb1, 2025.
- Metzger C., et al. Two-year Outcomes Using Fast-Acting Sub-perception Therapy for Spinal Cord Stimulation: Results of a Real-World Multicenter Study in the United States. Expert Rev Med Devices. Published online January 2025.
- Bayerl S, Paz-Solis J, Matis G, et al. Two-year outcomes using fast-acting, sub-perception therapy for spinal cord stimulation: a European, real-world, multicenter experience. JCM. 2024;13(22):6999
- Ferro R, North J, Kranenburg A, et al. Automated neural dosing for fast-acting subperception therapy: sustained spinal cord stimulation outcomes from a multicenter study. Pain Ther. 2025;14(4):1417-1429.