Boston Scientific accounts are for healthcare professionals only.
Profound. Proven. Different.
When you’ve done everything right but aren’t getting the results you want for your patient, try something DIFFERENT. Using the first and only FDA-approved MRI conditional adapters, connect other manufacturers’ leads to Boston Scientific’s innovative SCS therapy.
Profound pain relief with FAST™ Therapy
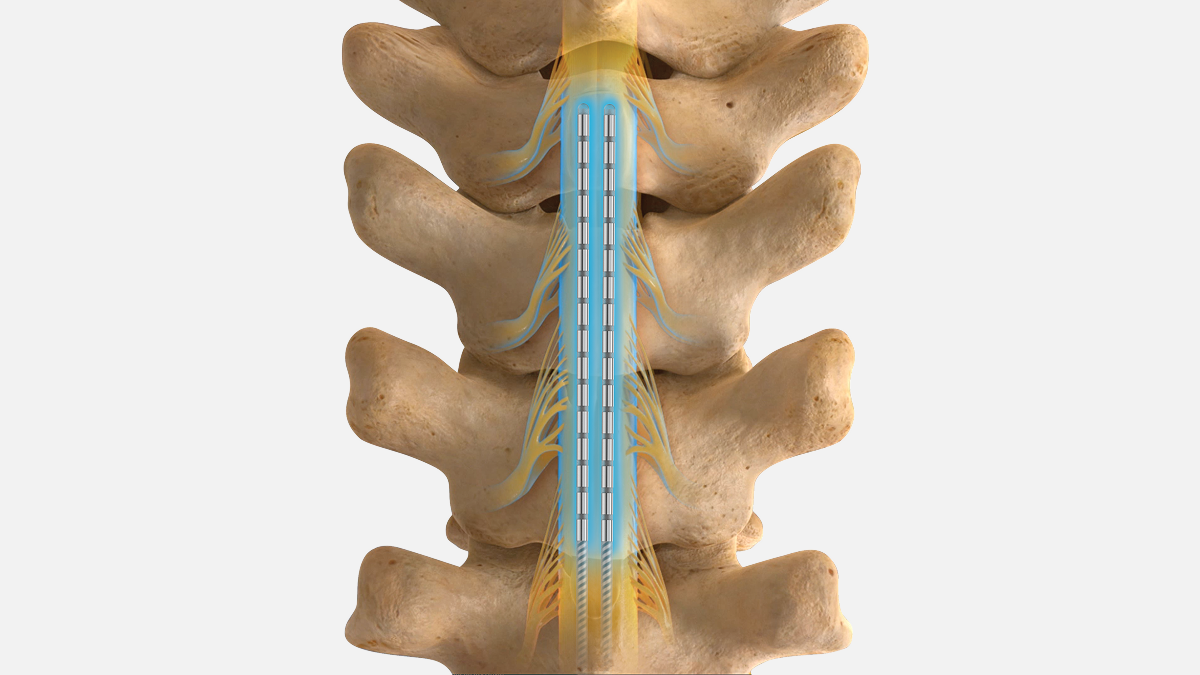
Our exclusive SCS FAST Therapy (Fast-Acting Sub-perception Therapy) delivers PROFOUND paresthesia-free pain relief in minutes that lasts for years.1-5

Proven SCS outcomes
Advanced SCS therapy options backed by robust clinical evidence
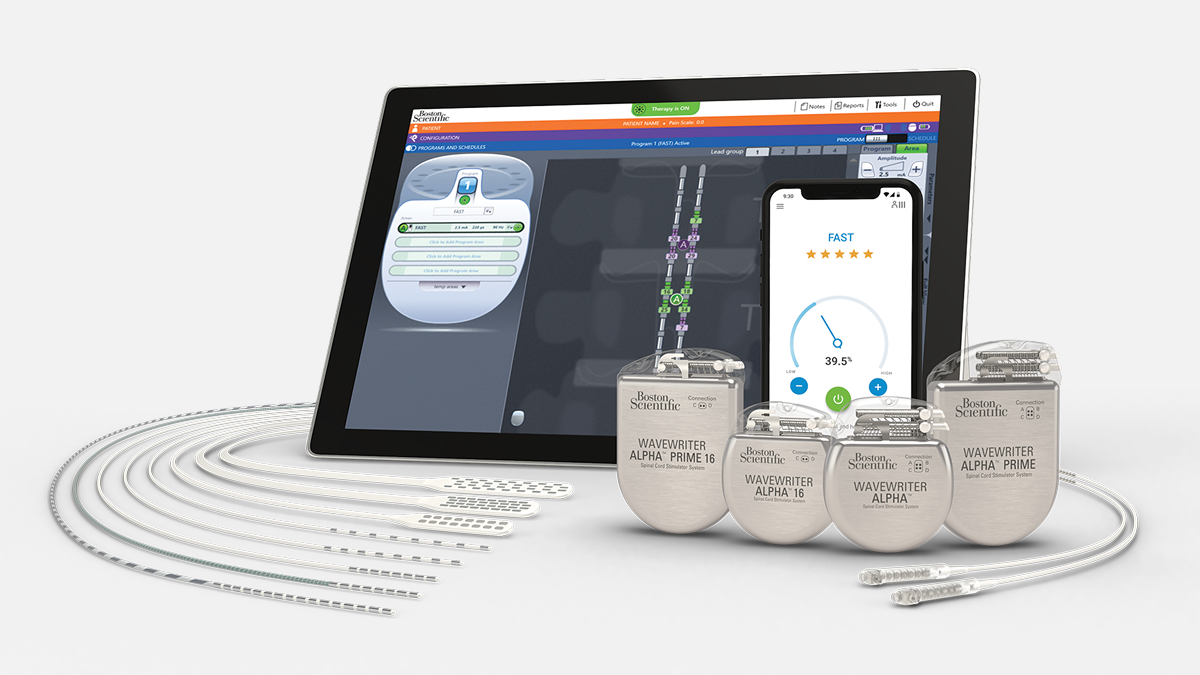
The WaveWriter Alpha SCS System offers all of Boston Scientific’s advanced therapies to provide a personalized treatment approach for each patient.
4 Level 1 randomized controlled trials and 4 real-world studies support the design, efficacy, and efficiency of Boston Scientific’s therapies.6-14

Different technology and outcomes
First and only MRI conditional adapters in SCS
Give your patients another option if their Abbott or Medtronic SCS therapy is no longer effective by connecting to Boston Scientific.‡ With MRI conditional adapters, patients maintain full-body MRI access.*
Boston Scientific's SCS therapy enables 60% salvage rate for failed trials and implants.15,16

Integrate the WaveWriter Alpha SCS System into your practice
For a list of compatible mobile devices, visit www.myscsjourney.com/mySCSGO. The mySCS™ GO Therapy Controller Application is compatible with the WaveWriter Alpha Spinal Cord Stimulation System only.
*MRI Conditional under specified conditions
†Disclaimer: These outcomes reflect approximated data as determined in the referenced studies.
‡ Refer to the Precision M8 Adapter Directions for Use for indications, warnings and cautions. The Precision M8 Adapter is compatible with the following Medtronic lead and extension model numbers: 39286, 39565, 3778, 3878, 3777, 3877, 3776, 3876, 3874, 3873, 3875, 37081, 37083, 37082, 977A160, 977A175, 977A190, 977A260, 977A275, 977A290, 977D160, 977D260, 977C165, 977C265, 977C190, 977C290
Refer to the “Precision™ S8 Adapter Directions for Use” for indications, warnings and cautions. The Precision™ S8 Adapter is compatible with the following St Jude Lead, Adapter and Extension model numbers: 3283, 3268, 3269, 3280, 3244, 3262, 3263, 3245, 3264, 3265, 3208, 3210, 3288, 3289, 3214, 3219, 3228, 3183, 3186, 3189, 3191, 3086, 3286, 2283, 3341, 3342, 3343, 3346, 3382, 3383, 3386.
Sub-perception stimulation has been demonstrated to be safe and effective in patients who have been treated successfully with conventional, paresthesia-inducing stimulation for at least six months. Full stimulation parameter ranges and options for both paresthesia based and sub-perception therapy are available for clinician’s use throughout the patient’s experience and treatment with SCS.
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
- Gage E., et al. Rapid Onset of Analgesia during Trial Period Utilizing Fast-Acting Sub-Perception Therapy SCS [Abstract] NANS 2023 Annual Meeting, Jan 12-15, 2023. (n=44)
- Anitescu M., et al. Utilizing SCS Designed to Engage Surround Inhibition Using Fast-Acting Sub-Perception Therapy (FAST): Prospective, Multicenter, Long-Term Outcomes [Abstract] NANS 2025 Annual Meeting, Jan 30-Feb 1, 2025. (n=20)
- Metzger C., et al. Two-year Outcomes Using Fast-Acting Sub-perception Therapy for Spinal Cord Stimulation: Results of a Real-World Multicenter Study in the United States. Expert Rev Med Devices. Published online January 2025. (n=50)
- Bayerl S, Paz-Solis J, Matis G, et al. Two-year outcomes using fast-acting, sub-perception therapy for spinal cord stimulation: a European, real-world, multicenter experience. JCM. 2024;13(22):6999. (n=52)
- Ferro R. et al. Streamlining Spinal Cord Stimulation Therapy via Personalized Automation of Programming [Abstract] NANS 2025 Annual Meeting, Jan 30-Feb 1, 2025. (n=23)
- Thomson SJ, Tavakkolizadeh M, Love-Jones S, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation; results of the PROCO randomized controlled trial. Neuromodulation. 2018;21(1):67-76. (n=20)
- Paz-Solis J. Thomson S, Jain R. Chen L, Huertas I, Doan Q. Exploration of high- and low-frequency options for subperception spinal cord stimulation using neural dosing parameter relationships: the HALO study. Neuromodulation, 2022;25(1):94-102. (n=30)
- North J, Loudermilk E, Lee A, et al. Outcomes of a multicenter, prospective, crossover, randomized controlled trial evaluating subperception spinal cord stimulation at ≤1.2 khz in previously implanted subjects. Neuromodulation. 2020:23(1):102-108. (n=140)
- Veizi E, Hayek SM, North J, et al. Spinal cord stimulation (SCS) with anatomically guided (3d) neural targeting shows superior chronic axial low back pain relief compared to traditional scs-lumina study. Pain Med. 2017;18(8):1534-1548. (n=169)
- Wallace MS, North JM, Phillips GM, et al. Combination therapy with simultaneous delivery of spinal cord stimulation modalities: COMBO randomized controlled trial. Pain Manag. 2023:13(3):171-184. (n=59)
- Metzger CS, Hammond MB, Pyles ST, et al. Pain relief outcomes using an SCS device capable of delivering combination therapy with advanced waveforms and field shapes, Expert Rev Med Devices. 2020;17(9):951-957. (n=84)
- Metzger C, et al. Long-Term Chronic Pain-Relief Outcomes Using an SCS System Capable of Combination Therapy (WaveWriter Outcomes Study] [Abstract]. Twenty-fifth Annual Meeting of the North American Neuromodulation Society, January 13-15th, 2022. (n=84)
- North J., et al. Evaluating SCS and Medical Management for Chronic Pain Without Prior Surgery: SOLIS RCT 24-Month Outcomes [Abstract], NANS 2025 Annual Meeting, Jan 30- Feb l, 2025. (n=77)
- Rauck R. et al. Real-World Outcomes Using Spinal Cord Stimulation for Treatment of Chronic Back Pain with No Prior Back Surgery [Abstract 88). Third joint Congress of the INS European Chapters, August 31-September 2nd, 2023, (n=48)
- Haider N, Ligham D, Quave B, et al. Spinal cord stimulation (SCS) trial outcomes after conversion to a multiple waveform SCS system. Neuromodulation. 2018:21(5):504-507. (n=16)
- Rigoard P. Billot M. Bougeard R, et al. Improved outcomes and therapy longevity after salvage using a novel spinal cord stimulation system for chronic pain: multicenter, observational, European case series. J Clin Med. 2024;13(4):1079. (n=43)
Warning: Stimulation modes. Only paresthesia-based stimulation mode has been evaluated for effectiveness in the diabetic peripheral neuropathy (DPN) population.
View Boston Scientific Spinal Cord Stimulator System Indications, Safety, and Warnings