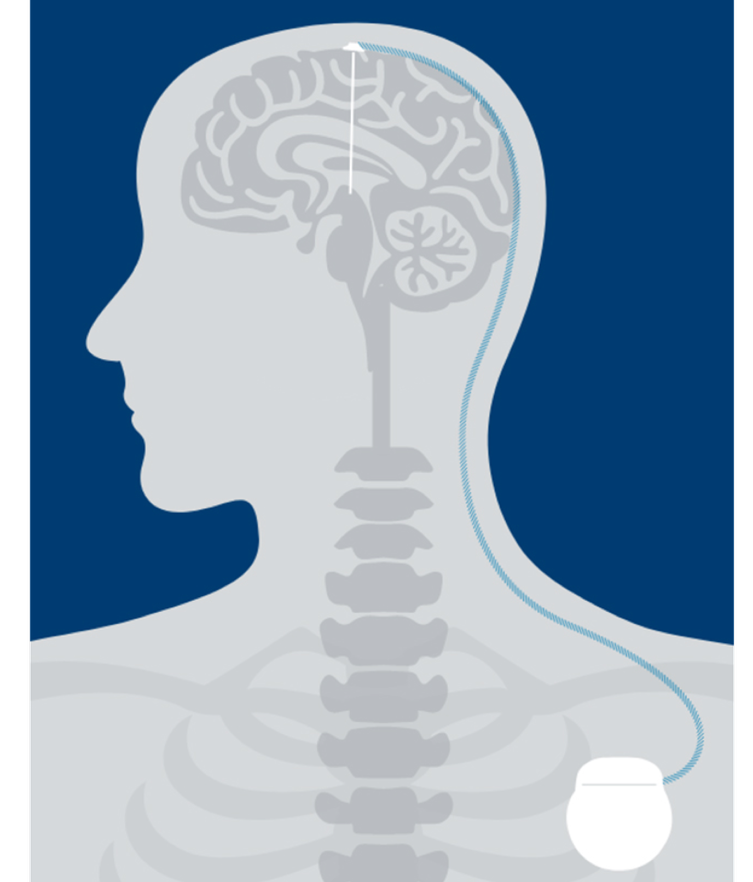
People living with Parkinson’s Disease (PD) or essential tremor (ET) experience a disturbance in motor symptoms because of low dopamine levels in the brain causing abnormal signaling. DBS can help regulate those signals through electrical stimulation. As a result, symptoms are often reduced.1
Leads
Your doctor will place one or two insulated wires called “leads” in the brain, which connect to a thin wire called an “extension.”

Stimulator
A small device called a “stimulator” is implanted under the skin in the chest, which also connects to the extension.

Therapy
The stimulator sends mild electrical pulses through the extensions and leads to specific regions of the brain.

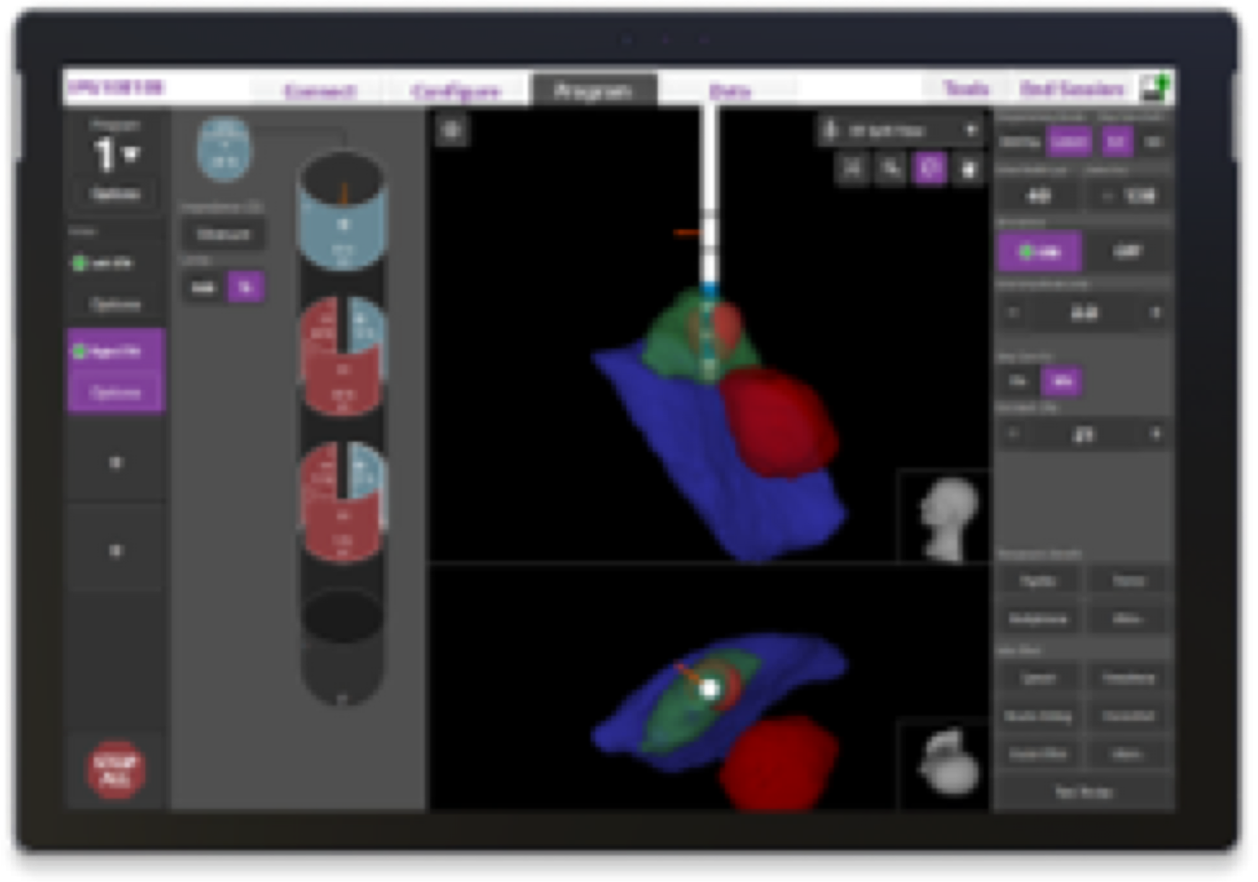
Programming
Your device is programmed with Image Guided Programming; a technology that allows your physician to see exactly where your leads are placed and exactly where the therapy is being delivered.
Benefits of DBS
DBS has changed the lives of people with PD, easing symptoms and returning function that was once lost. The results of DBS vary from person to person depending on the overall level of health, progression, and window of implementation.
Benefits of DBS include
- DBS is a safe and effective solution to manage and reduce symptoms such as: tremor, slowness, rigidity, uncontrolled movements, incontinence, and moodiness
- Your initial programming session may be 20 minutes shorter than a typical programming session with Image Guided Programming²
- For Parkinson’s Disease (PD) patients:
- May allow for a reduction in number of medications taken³
- Provide 6 hours of more “On Time”, giving you control and independence to live your life free of rigidity, freezing or the troublesome dyskinesia sometimes caused by medications⁴
- Patients with tremor experienced an average of 70% reduction of symptoms, depending on type and location⁵
- Patients showed marked improvements in minor functions and sustained improvement for at least 5 years⁶
- For Essential Tremor (ET) patients:
- According to research, patients exhibit 60-90% improvement of tremor⁷
- 98% of patients receiving DBS therapy for Essential Tremor would recommend it to others⁸

Know the timing of DBS
There is an ideal “window” for people with PD to consider DBS surgery. In general, it’s best to consider DBS when you’re still responding to levodopa, but you’re no longer able to control your motor symptoms with medication alone. This timing will differ from patient to patient, as the path and progression of Parkinson’s is personal. Talk to your doctor in the early stages of treatment, and voice your desire to explore alternatives like DBS as you move through your journey.5

“Thanks to DBS, my husband Kenny has experienced a drastic improvement in his symptoms and reduction in his medicine. That was a huge relief.”
- Tania—Caregiver to Kenny, a Boston Scientific DBS patient*
When DBS is most effective⁶
Remember, PD varies from person to person.
There is no age cut-off for DBS, yet overall health status will influence if you are a good candidate or not.
You are still responding positively to Parkinson’s Disease medications (levodopa) but are no longer able to control motor symptoms with only medications.
Signs and evidence of dementia may influence if a specialist recommends you for DBS.
The best way to control any disease progression is to be proactive and start talking to your care team now. A DBS specialist could help you.